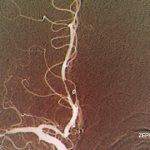
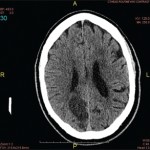
The overlapping clinical and imaging features of inflammatory CAA with other inflammatory and non-inflammatory conditions lead to a broad and challenging differential diagnosis.3-6 Histological sampling in the form of stereotactic brain biopsy remains the gold standard for diagnosing inflammatory CAA.
The recognition that typical MRI findings in the appropriate clinical context can indicate the diagnosis of inflammatory CAA prompted the development and validation of diagnostic clinical-radiological criteria. These criteria consider the patient’s age, clinical presentation, white matter hyperintensities and hemorrhagic lesions to classify patients into probable and possible CAAri, avoiding the risks and complications associated with an invasive diagnostic procedure.23 Of note, only patients with the pathologic subtype of CAAri were included in this study. Based on this algorithm, our patient would have been classified with probable CAAri despite the final pathology confirming the presence of the ABRA subtype. This corroborates the notion that the two pathological subtypes of CAA share many clinical and radiological features.
Regarding ancillary tests that can aid in the diagnosis of ABRA, cerebrospinal fluid examination reflects non-specific inflammatory changes, including elevated protein (71%) and lymphocytic pleocytosis (44%).8 Serum inflammatory markers, including CRP and ESR, were elevated in 60% and 37.5% of cases, respectively, in a recent systemic review that included patients with both CAAri and ABRA.24
No systematic evidence evaluating treatment strategies for ABRA or CAAri exists, and most recommendations are borrowed from experience with primary angiitis of the CNS (PACNS). Nevertheless, the effectiveness of immunosuppressive therapy has been confirmed in sizeable patient series in daily practice.25
Chu et al. noticed that 78% of the ABRA patients in their series showed complete or partial response to glucocorticoids.8 High-dose glucocorticoids are typically used, including intravenous 1 g/day methylprednisolone for three to five days, followed by a prolonged tapering dose of oral glucocorticoids.
In the same study and other case series, multiple immunomodulatory agents have been implemented as steroid-sparing agents, including cyclophosphamide, mycophenolate mofetil, methotrexate and azathioprine.8,20,21,24
Caldas et al. noticed that within 21 months, 42% of patients with inflammatory CAA returned to their baseline functional status, 37% died and 20% still had residual functional disabilities following treatment. No difference in outcomes between patients treated with glucocorticoids alone, or in combination with other agents, was noted.24 Interestingly, as demonstrated by Salvarani et al., a subset of patients with prominent leptomeningeal enhancement shows a favorable response to therapy.26
Conclusions & Future Directions
Despite our progress in understanding and managing ABRA, many gaps in knowledge remain. Expanding and validating the proposed clinical-radiological criteria on ABRA cases could further reduce the need for invasive diagnostic tests; identifying subgroups of patients within the spectrum of inflammatory CAA who would need more aggressive management could also be an area of future research; the development of formal treatment guidelines; and consideration of agents used in other granulomatous inflammatory diseases, such as tumor necrosis factor alpha (TNFα) inhibitors could also be included in the future research agenda.