Approximately one month later, she was readmitted to the hospital because of dizziness and light-headedness while sitting in her car, and her ECG on readmission showed a narrow complex tachycardia with non-sinus atrial activity, interpreted as possible atrial tachycardia with atypical atrial flutter. She remained hemodynamically stable, and her symptoms resolved with carvedilol, so she was discharged home after a short hospitalization.
A few weeks later, she was readmitted once again after her external defibrillator vest delivered a shock during continued intermittent palpitations at home, and review of the external defibrillator vest recordings showed supraventricular tachycardia in the 200 bpm range. Repeat ECG was interpreted as atrioventricular nodal re-entrant tachycardia. The electrophysiology team was consulted and performed color mapping, showing areas of scar tissue or fibrosis (see Figure 4). Activation mapping was also done, which revealed the location of an atrial micro-reentry circuit, resulting in atrial flutter (see Figure 5), in the same location as the fibrosis. The electrophysiologist successfully ablated this area, resolving the patient’s recurrent arrhythmias.
Her final discharge diagnosis was recurrent atrial re-entry tachycardia secondary to primary cardiac fibrosis from SSc. At her follow-up visit with a rheumatologist, the patient was feeling well without any symptoms of palpitations, presyncope, SOB or volume overload. She did not require any long-term anti-arrhythmic medication.
Awareness of cardiac manifestation is paramount, because cardiac involvement portends a poor prognosis in SSc.
Discussion
Clinical cardiac manifestations occur in 15–35% of scleroderma (SSc) patients, but the majority of SSc patients have subclinical cardiac involvement at post-mortem autopsy.1,2 Awareness of cardiac manifestation is paramount, because cardiac involvement portends a poor prognosis in SSc.2 Primary cardiac manifestations include fibrosis of the myocardium, pericardium and the conduction system, as well as heart failure and arrhythmia.1 Secondary cardiac manifestations occur secondary to pulmonary hypertension, interstitial lung disease or renal failure resulting in cardio-renal syndrome.1
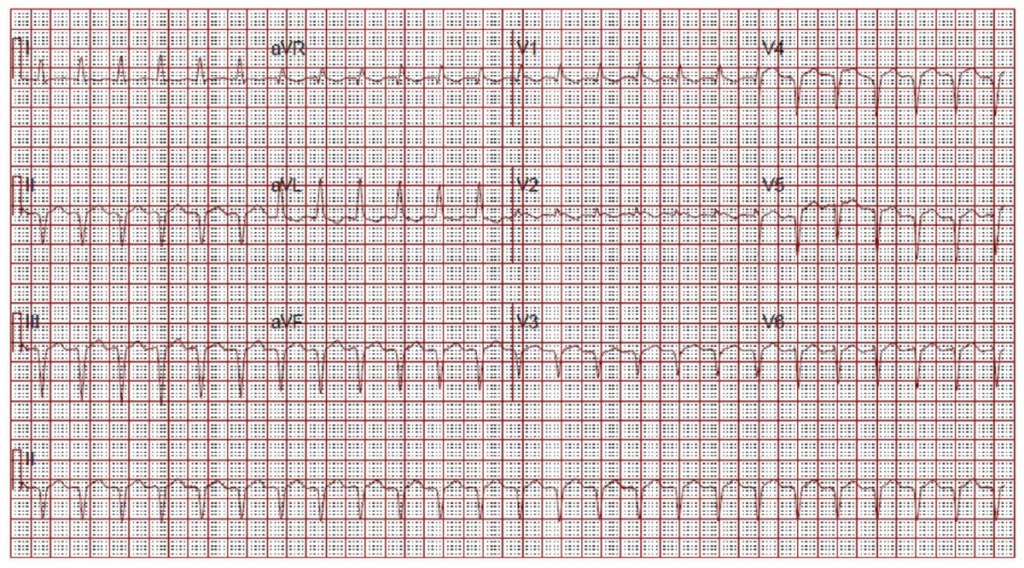
(click for larger image) Figure 2: ECG on Admission
Narrow complex tachycardia. Interpreted as sinus tachycardia.
Primary injury to an organ results in initiation of a fibrogenic response, activation of effector cells, elaboration of extracellular matrix (ECM) and dynamic deposition with insufficient resorption of ECM.3 This is no different when it comes to the heart.
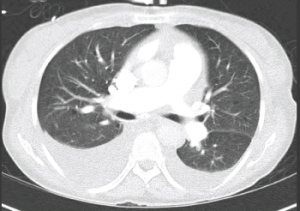
Figure 3: CT Chest
Bilateral pleural effusions. No evidence of pulmonary embolism or cardiomegaly.
As with most organs in the body, the balance of normal architecture is key in normal functioning. Cardiac cells are embedded in a collagen network made up of mostly type I (80%) and type III collagen (11%).4 This collagen network provides physical support, and also sustains transmission of evenly distributed force throughout the myocardium.4 Connexins also play a major role in propagation of electrical impulse through the heart.4 Cardiac fibroblasts are the most abundant cell type in the myocardium.3 These cells are derived from fibroblasts that are native to the myocardium, circulating in the blood or result from epithelial to mesenchymal transition (EMT).3
