Scleromyxedema is a primary cutaneous mucinosis characterized by a diffuse and generalized papular skin eruption of mucinous deposits throughout the upper dermis. In addition to dermatologic manifestations, scleromyxedema may involve the cardiopulmonary, gastrointestinal, renal and nervous systems.
Dermato-neuro syndrome (DNS) is a rare, severe neurologic complication of scleromyxedema.1,2 The pathogenesis of DNS is unknown, but circulating cytokines may play a role. With intravenous immunoglobulin (IVIG) maintenance therapy, recurrence is rare.
Coronavirus disease 2019 (COVID‑19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily causes respiratory problems; however, central nervous system (CNS) manifestations have also been reported. Neurological complications of COVID-19 include headache, encephalopathy, hyposmia and dysgeusia.3 Multiple mechanisms contribute to neurologic complications of COVID-19, including coagulopathy, inflammatory vasculopathy and cytokine release syndrome.
We present the case of a patient with well-controlled scleromyxedema on maintenance IVIG therapy who had DNS recurrence secondary to COVID-19, illustrating the potential role of SARS-CoV-2 in immune dysfunction and the pathogenesis of DNS.
Case Presentation
A 58-year-old woman with well-controlled scleromyxedema and a prior episode of DNS, who was being treated with levetiracetam and IVIG maintenance therapy without interruption in therapy, presented with encephalopathy and a one-week history of shortness of breath and upper respiratory symptoms. The examination was remarkable for disorientation, generalized decreased motor strength and diffusely tight and hypertrophic skin compared with baseline. She had intermittent rhythmic right-arm shaking episodes that generalized into prolonged tonic-clonic convulsions with associated urinary incontinence.
Continuous electroencephalogram showed non-convulsive status epilepticus (NCSE) that progressed to super-refractory NCSE (see Figure 1). The patient was intubated and treated with levetiracetam (60 mg/kg intravenous load, followed by 2,000 mg twice daily), fosphenytoin (20 mg/kg intravenous load, followed by 200 mg twice daily), lacosamide (300 mg intravenous load, followed by 200 mg twice daily), phenobarbital (15 mg/kg intravenous load, followed by 100 mg twice daily), and midazolam (0.2 mg/kg intravenously administered, followed by continuous infusion with repeat loading dose every five minutes to a maximum total dose of 1 mg/kg) and pentobarbital infusion (15 mg/kg administered at a rate of ≤50 mg/minute, followed by 1 mg/kg/hour continuous infusion to achieve burst suppression for 48 hours, followed by gradual withdrawal within 24 hours to prevent recurrent status epilepticus).
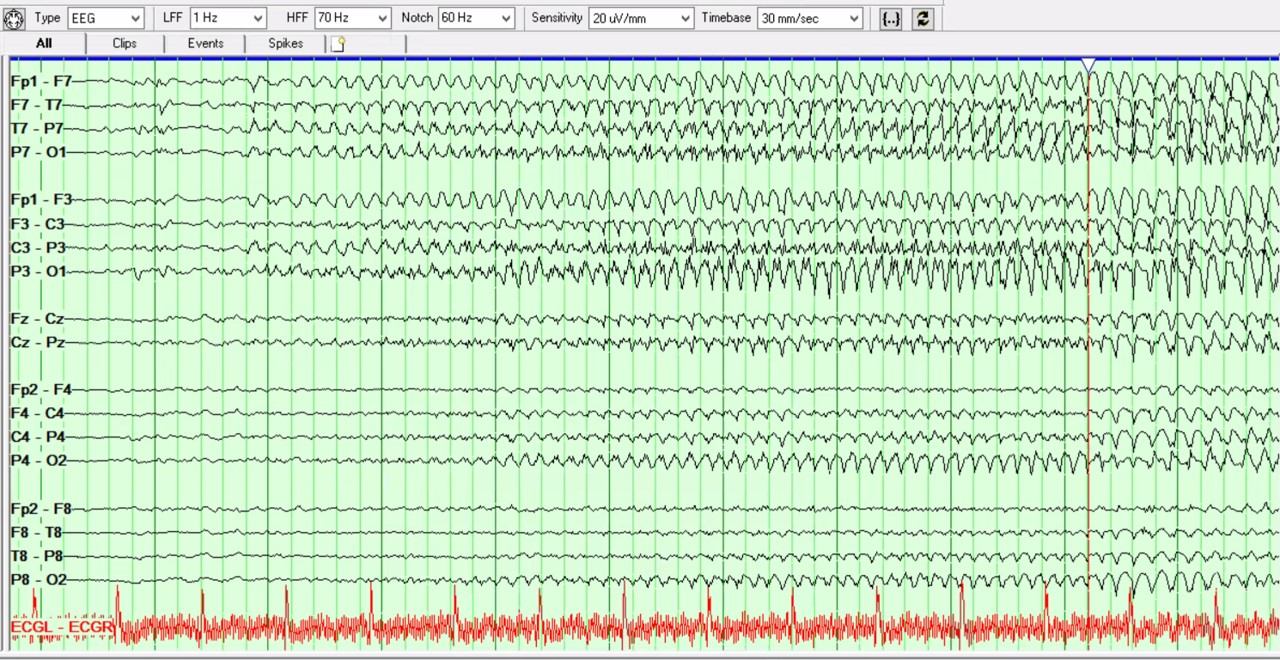
This bipolar montage shows a seizure arising from burst activity, with spikes over the
left hemisphere, evolving into the left more than the right hemisphere, with bilateral,
rhythmic, sharply contoured theta activity. The EEG shows further rhythmic, spike/
polyspike discharges until seizure termination.
A nasopharyngeal swab was positive for SARS-CoV-2 by real-time reverse transcription polymerase chain reaction (rRT-PCR) assay, and the patient was treated with remdesivir (200 mg intravenously on day 1, followed by 100 mg daily for 10 days).
Serum studies were notable for elevated C-reactive protein (CRP; 223.7 mg/L; reference range [RR]: <10.0 mg/L), D-dimer of 1,087 ng/mL; RR: <230 ng/mL), interleukin-6 (IL-6) of >400 pg/mL (RR: ≤1.8 pg/mL) and IgG levels of 3,347 mg/dL (RR: 600–1,700 mg/dL). Tests for human immunodeficiency virus and rapid plasma reagin were negative.
The patient’s cerebrospinal fluid (CSF) was significant for lymphocytic pleocytosis (183 cells/mm2; RR: 0–5 cells/mm2), but was within normal limits for protein and glucose. The tests did not show evidence of herpes simplex virus, varicella zoster virus, Epstein-Barr virus, arbovirus, enterovirus, West Nile virus or Borrelia burgdorferi. The autoimmune encephalitis panel and bacterial and fungal cultures were negative. A PCR for SARS-CoV-2 was not obtained. Influenza A/B tests were negative.
Magnetic resonance imaging (MRI) of the brain showed T2/FLAIR (T2-weighted fluid-attenuated inversion recovery) hyperintensity in the left thalamus and occipital lobe with restricted diffusion (see Figure 2).
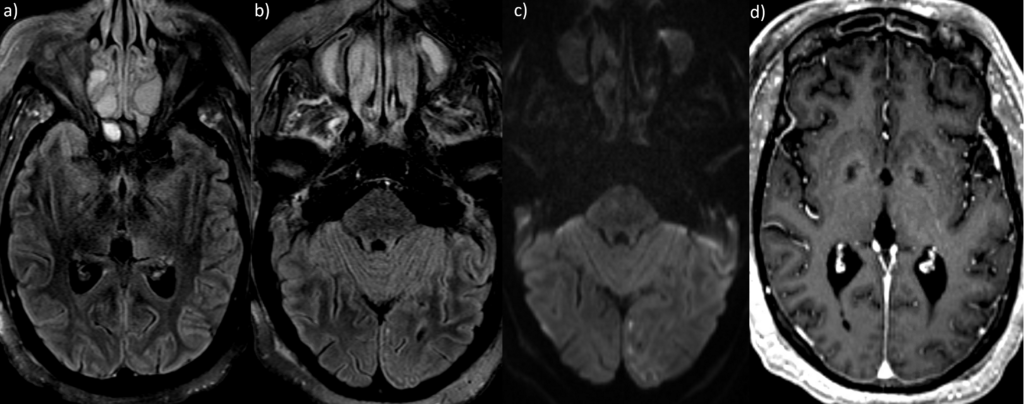
The T2/FLAIR MRI demonstrates hyperintensity in the left thalamus (a) and occipital lobe (b), with associated diffusion restriction (c).
An MRI with contrast demonstrates diffuse leptomeningeal enhancement, especially involving the left posterior parafalcine
occipital lobe (d).
A rheumatologist was consulted during her admission. Upon repeat physical examination, the patient appeared somnolent but arousable with generalized motor weakness. Her skin had diffuse thickening and multiple papular lesions of 1–2 mm in diameter over the forearms and anterior chest. Sclerodactyly was present, but without digital ulcers, telangiectasias or calcinosis. Diffuse alopecia was noted. She did not exhibit leonine facies, malar rash or discoid lesions. The musculoskeletal exam was notable for the lack of synovitis or chronic arthropathic changes of the small joints of her hands, wrists, elbows, knees, ankles or feet.
Her serologies failed to demonstrate evidence of rheumatoid factor, anti-cyclic citrullinated peptide antibodies, anti-neutrophil cytoplasmic antibodies, anti-nuclear antibodies, anti-double-stranded DNA antibodies, anti-Smith antibodies, anti-ribonuclear ribonucleoprotein antibody, anti-Sjögren’s-syndrome-related antigens (SSA and SSB), anti-Scl-70 antibodies, anti-ribosomal-P antibodies, anti-centromere antibodies and anti-Jo-1 antibodies. Additionally, her C3 and C4 complement levels were normal. Although the patient had notably elevated inflammatory markers at the time of her current presentation, this was attributable to her active SARS-CoV-2 infection.
Diagnostic considerations for an encephalopathic patient with pleocytosis on CSF would have included CNS infections, malignancies and autoimmune conditions, such as autoimmune encephalitis, and connective tissue diseases, such as systemic lupus erythematosus (SLE) or primary Sjögren’s syndrome with CNS involvement, but this patient had a well-established diagnosis of scleromyxedema with an extensive negative evaluation that was confirmed in 2015 at another institution and was previously reported in the literature in 2017.4
Because she did not meet clinical or serologic criteria for either SLE or primary Sjögren’s syndrome, and her CSF evaluation failed to demonstrate evidence of an infectious etiology (other than SARS-CoV-2), recurrence of DNS in the setting of scleromyxedema was thought to be consistent with her clinical presentation.
The patient was diagnosed with DNS in the setting of SARS-CoV-2 infection, and the patient received two, five-day courses of IVIG and 1 g of intravenous methylprednisolone daily for three days followed by a prednisone taper. After the second course of IVIG treatment, the patient regained baseline mental status, and repeat inflammatory markers, including CRP, D-dimer and IL-6 (5.6 pg/mL), trended down to normal limits.
Discussion
Scleromyxedema is a rare disease characterized by generalized papular eruptions, mucin deposition in the dermis and paraproteinemia. Multiple systemic and neurological manifestations of scleromyxedema have been reported.4 Neurologic manifestations of scleromyxedema can range from confusion to fatal encephalopathy.5
In 1996, River et al. coined the term dermato-neuro syndrome to describe a triad of fever, convulsions and coma with sclerodermoid skin changes, and since then, a few case reports have been published.1,4-6 DNS is a rare, severe complication of scleromyxedema with a mortality rate up to 21%.1,7
To date, the pathophysiology of DNS is not well understood, but a monoclonal paraproteinemia, most commonly IgG, likely plays a fundamental role in the pathogenesis of this disorder.1 However, the level of paraproteinemia does not always correlate with the severity of the disease.
Another hypothesis is that IgG crossing the blood-brain barrier (BBB) is mediated by increased IL-6 production, which supports the coexistence of a secondary mechanism that facilitates IgG neurotoxicity mediated by IL-6 given its potential in BBB permeability.1,8,9 Elevated CSF and serum IL-6 have been reported in previous cases of DNS, and IL-6 was implicated as a potential mechanism of injury in a patient with influenza A-triggered DNS.10,11
SARS-CoV-2 has caused a rampant pandemic. The virus mainly affects the lower respiratory tract. Neurologic symptoms can include headache, dizziness, altered mental status, and smell and taste disorders. Severe complications, such as strokes and neuromuscular and seizure disorders, have also been reported.3 Critically ill patients who require care in the intensive care setting have a higher occurrence of neurologic complications than less severely ill patients.12
SARS-CoV-2 may induce a cytokine storm that, in severe cases, can lead to multi-organ failure and fatalities. This cytokine storm is characterized by an increase in IL-1, IL-2, IL-6, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, macrophage inflammatory protein 1α and tumor necrosis factor (TNF) α.13-15
A mouse model showed that SARS-CoV-2 neurovirulence was due to the virus’ ability to induce local proinflammatory cytokine production by astrocytes and microglia.16 In vitro exposure to COVID-19 causes glial cells to secrete IL-6, IL-12, IL-15 and TNFα, which is supported by elevated CSF IL-6 levels reported in patients with SARS-CoV-2 encephalitis.17,18
Another proposed mechanism of CNS injury is direct viral-mediated injury by the SARS-CoV-2 virus, either via a leaky BBB from pro-inflammatory cytokines or endothelial angiotensin converting enzyme-2 (ACE-2) receptors in the central nervous system. In cases of COVID-19-related encephalitis with negative PCR in cerebrospinal fluid, cytokine storm is thought to be mechanism of CNS injury.15
Although CSF IL-6 was not measured in our patient, our patient’s IgG and IL-6 serum levels were elevated, suggesting a mechanism that facilitated IgG neurotoxicity via IL-6-induced blood-brain barrier permeability. This evidence suggests an overlap in COVID-19 and DNS’ pathophysiology that could have triggered the second episode of DNS despite well-controlled scleromyxedema.
This may be the first case of DNS associated with SARS-CoV-2 infection. In 2016, Bhoyrul et al. reported a case of influenza trigger for DNS, but the case was not associated with DNS recurrence on therapy.9 To our knowledge, this is the third case report of DNS recurrence and the first reported case of DNS recurrence secondary to a viral infection, specifically SARS-CoV-2 infection.2,7
The acute onset of DNS in the context of elevated inflammatory markers and normalization of inflammatory markers with subsequent clinical improvement following immunotherapy leads us to hypothesize that our patient had a para-infectious process secondary to SARS-CoV-2 infection that provoked DNS recurrence despite well-controlled scleromyxedema on maintenance IVIG therapy.
Our findings, together with the consistently reported respiratory symptoms preceding the onset of DNS, suggest that viral infections play a role in pathogenesis of DNS. This case also highlights that scleromyxedema patients are at risk of developing DNS or DNS recurrence during SARS-CoV-2 infection. Physicians should be aware of this complication and reinforce public health recommendations accordingly.
 Fazila Aseem, MD, MPH, is a clinical instructor in neurology at the University of North Carolina Hospital at Chapel Hill.
Fazila Aseem, MD, MPH, is a clinical instructor in neurology at the University of North Carolina Hospital at Chapel Hill.
 Alexander D. Jeffs, MD is a first-year orthopedics resident at the University of North Carolina at Chapel Hill. He completed his medical school at UNC and chose to participate in interdisciplinary critical care medicine.
Alexander D. Jeffs, MD is a first-year orthopedics resident at the University of North Carolina at Chapel Hill. He completed his medical school at UNC and chose to participate in interdisciplinary critical care medicine.
 Enid Y. Sun, MD, MPH, is an assistant professor in rheumatology at Temple University. She completed her residency and fellowship in rheumatology at the University of North Carolina at Chapel Hill.
Enid Y. Sun, MD, MPH, is an assistant professor in rheumatology at Temple University. She completed her residency and fellowship in rheumatology at the University of North Carolina at Chapel Hill.
 Randaline R. Barnett, MD, is a sixth-year neurosurgery resident at the University of North Carolina at Chapel Hill. She is planning to pursue a fellowship in pediatric neurosurgery.
Randaline R. Barnett, MD, is a sixth-year neurosurgery resident at the University of North Carolina at Chapel Hill. She is planning to pursue a fellowship in pediatric neurosurgery.
 Courtney Blodgett, AG-ACNP, is an acute care nurse practitioner at the University of North Carolina at Chapel Hill.
Courtney Blodgett, AG-ACNP, is an acute care nurse practitioner at the University of North Carolina at Chapel Hill.
 Winnie Lau, MD, is assistant professor of neurology at the University of North Carolina at Chapel Hill. She is a clinician educator.
Winnie Lau, MD, is assistant professor of neurology at the University of North Carolina at Chapel Hill. She is a clinician educator.
 Casey Olm-Shipman, MD, MS, is assistant professor of neurology and director of quality improvement at the University of North Carolina at Chapel Hill.
Casey Olm-Shipman, MD, MS, is assistant professor of neurology and director of quality improvement at the University of North Carolina at Chapel Hill.
 Matthew F. Sharrock, MD, is assistant professor of neurology and medical director of the Neuro Intensive Care Unit at the University of North Carolina at Chapel Hill.
Matthew F. Sharrock, MD, is assistant professor of neurology and medical director of the Neuro Intensive Care Unit at the University of North Carolina at Chapel Hill.
 Rhonda Cadena, MD, is assistant professor of neurology and emergency medicine at the University of North Carolina at Chapel Hill.
Rhonda Cadena, MD, is assistant professor of neurology and emergency medicine at the University of North Carolina at Chapel Hill.
 Yueh. Z. Lee, MD, PhD, is an associate professor of radiology-neuroradiology at the University of North Carolina at Chapel Hill.
Yueh. Z. Lee, MD, PhD, is an associate professor of radiology-neuroradiology at the University of North Carolina at Chapel Hill.
 Alfredo C. Rivadeneira, MD, is a professor of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill.
Alfredo C. Rivadeneira, MD, is a professor of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill.
 Clio A. Rubinos, MD, MS, is an assistant professor of neurology and the director of the post-acute symptomatic seizure (PASS) clinic, co-director of the global health neurology elective rotation, and the Neurocritical Care Fellowship Program director at the University of North Carolina at Chapel Hill.
Clio A. Rubinos, MD, MS, is an assistant professor of neurology and the director of the post-acute symptomatic seizure (PASS) clinic, co-director of the global health neurology elective rotation, and the Neurocritical Care Fellowship Program director at the University of North Carolina at Chapel Hill.
References
- Fleming KE, Virmani D, Sutton E, et al. Scleromyxedema and the dermato-neuro syndrome: Case report and review of the literature. J Cutan Pathol. 2012 May;39(5):508–517.
- Gonzalez J, Palangio M, Schwartz J, et al. Scleromyxedema with dermato-neuro syndrome. J Am Acad Dermatol. 2000 May;42(5 Pt 2):927–928.
- Divani AA, Andalib S, Biller J, et al. Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2020 Oct 30;20(12):60.
- Weiner J, Marano A, Cardones A, et al. Fever, joint pain, seizures, and rash in a 53-year-old woman. Arthritis Care Res (Hoboken). 2017 Sep; 69(9):1437–1443.
- Berger JR, Dobbs MR, Terhune MH, Maragos WF. The neurologic complications of scleromyxedema. Medicine (Baltimore). 2001 Sep; 80(5):313–319.
- River Y, Levy I, Gilead L, et al. Fever, convulsions and coma in scleromyxedema: A ‘dermato-neuro syndrome.’ Neurology. 1996 Jun;46(6):1778–1779.
- Liu A, Suozzi K, Hwang DY, et al. Dermatoneuro syndrome A full recovery after a second episode. Neurol Clin Pract. 2016 Jun;6(3):e27–e29.
- Rochfort KD, Collins LE, Murphy RP, Cummins PM. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: Consequences for interendothelial adherens and tight junctions. PLoS One. 2014 Jul 3;9(7):e101815.
- Marshall K, Klepeiss SA, Ioffreda MD, Helm KF. Scleromyxedema presenting with neurologic symptoms: A case report and review of the literature. Cutis. 2010 Mar;85(3):137–140.
- Bhoyrul B, Mughal AA, Paulus J, et al. Does dermatoneuro syndrome have a viral aetiology? Clin Exp Dermatol. 2016 Jan;41(1):53–56.
- Johkura K, Susuki K, Hasegawa O, et al. Encephalopathy in scleromyxedema. Neurology. 1999 Sep 22;53(5):1138–1140.
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020 Jun 1;77(6):683–690.
- Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020 Dec;9(1):1123–1130.
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020 Jul 28;71(15):762–768.
- Bodro M, Compta Y, Sánchez-Valle R. Presentations and mechanisms of CNS disorders related to COVID-19. Neurol Neuroimmunol Neuroinflamm. 2020 Dec 11;8(1):e923.
- Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol. 2004 Apr;78(7):3398–3406.
- Bodro M, Compta Y, Llansó L, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS- CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020 Jul 1; 7(5):e821.
- Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018 Oct 26; 12:386.