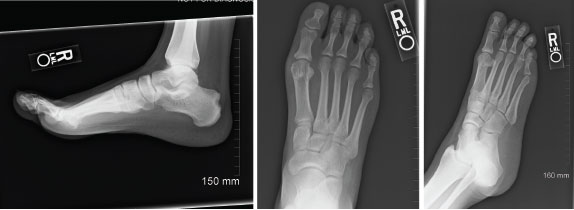
Figure 1: Radiographs of Right Foot, 2012
Imaging demonstrates a large erosion involving the posterior margin of the right calcaneus, near the Achilles insertion. Erosive changes involving the first metatarsophalangeal joints are noted, consistent with an inflammatory arthritis.
The nephrologist believed a diagnosis of Gitelman syndrome was appropriate based on her metabolic findings at that time.
She tested negative for rheumatoid factor and anti-citrullinated peptide antibodies, and a uric acid level of 10.9 mg/dL was documented. Imaging of her feet revealed erosions in both first MTP joints and a large right calcaneal erosion (see Figure 1). Conventional radiographic imaging revealed right-sided sacroiliitis, although she had not complained of back pain.
At that time, she was felt to have an inflammatory, erosive arthritis, and in combination with the radiographic findings of sacroiliitis, a diagnosis of spondyloarthropathy was made. She was started on a tumor necrosis factor-α inhibitor.
Four years later, in 2016, the patient was hospitalized with polyarthritis. A right knee arthrocentesis was performed, revealing intra- and extracellular monosodium urate crystals. Around this same time, tophi of the elbows and third distal interphalangeal joint were reported.
For the first time, this 27-year-old premenopausal female had a crystal-proven diagnosis of tophaceous gouty arthritis. She was treated with corticosteroids and discharged from the hospital. On a subsequent outpatient visit, 100 mg of daily allopurinol was initiated, with a concomitant prednisone taper.
One month later the patient was hospitalized in the intensive care unit with DRESS (i.e., drug reaction with eosinophilia and systemic symptoms), based on fever, rash and time frame of recently starting allopurinol, which was subsequently discontinued.
Shortly thereafter, her uric acid level was elevated at 13.2 mg/dL with a creatinine of 1.5 mg/dL and estimated GFR of 50. Febuxostat (40 mg per day) and colchicine (0.6 mg daily) were initiated. On subsequent follow-up visits, her uric acid levels did decline, but never below goal levels of <6g/dL, and over the next four years she continued to experience recurrent gout attacks.
At various times during 2016–2020, laboratory testing demonstrated hypercalcemia, with levels between 10.4 and 11.2 mg/dL. This was initially presumed to be due to secondary hyperparathyroidism from her chronic renal disease. In 2016, an endocrinologist made a diagnosis of primary hyperparathyroidism based on significantly elevated parathyroid hormone levels of 2,471 pg/mL at maximum (intact parathyroid hormone RR: 15–72 pg/mL). A parathyroid ultrasound revealed bilateral parathyroid adenomas. She underwent a parathyroidectomy in 2020.
Case Summary
The patient’s complete medical history included:
- Gitelman syndrome with chronic, stage IV kidney disease;
- Crystal-proven tophaceous gout, with recurrent attacks of gouty arthritis over many years;
- Possible spondyloarthropathy;
- Anemia due to chronic kidney disease;
- Obesity; and
- Primary hyperparathyroidism.
Her surgical history included a salpingostomy for an ectopic pregnancy in 2016 and the subtotal parathyroidectomy in 2020.
She is married with two children, and she denied any tobacco or alcohol use. Her family history was significant for fibromyalgia in her mother, but no family history of gout.
Her outpatient medications included 5 mg of amiloride daily, 500 mg of calcium carbonate, 2,850 mg of calcium citrate three times a day, 40 mg of febuxostat daily, 0.6 mg of colchicine three times per week and 50 mg/0.5 mL of golimumab via subcutaneous monthly injections. Current inpatient medications consisted of calcium gluconate and magnesium replacement.
Her vital signs from the current inpatient admission demonstrated a temperature of 99.4ºF and a blood pressure of 151/74 mmHg. A physical exam revealed an obese, uncomfortable-appearing woman with a warm right knee with a moderate effusion and very limited range of motion due to pain. Her left ankle was also warm and painful, with a reduced range of motion.
Her white blood cell count was 13.8K/uL, and her creatinine level was 2.75 mg/dL (eGFR 24). Two weeks previously, her uric acid level was 8.5 mg/dL, and that test was not repeated.
An arthrocentesis of the right knee was performed, yielding a total cell count of 35,800K/uL with 97% neutrophils and identification of negatively birefringent needle-shaped crystals consistent with gouty arthritis.
Treatment included a prolonged course of steroids and continuation of febuxostat. In the three months following her hospitalization and parathyroidectomy, she was hospitalized six more times with symptomatic hypocalcemia and recurrent gout attacks.
Discussion
Gitelman syndrome is a rare, autosomal recessive genetic defect of the sodium chloride transporter, resulting in a salt-wasting nephropathy with complications of hypomagnesemia, hypokalemia and hypocalciuria. The defective transport mimics that of a thiazide diuretic, targeting the distal tubule. This results in enhanced salt and hydrogen ion secretion, leading to metabolic alkalosis with stimulation of the renin-angiotensin-aldosterone system, and reabsorption of uric acid through the urate transporter URAT1.1-3
Additionally, defects in the uric acid transporter SLC17A3 have been described in Gitelman syndrome, and this may also contribute to hyperuricemia.2,4-6
Treatment for Gitelman syndrome consists of correcting the electrolyte derangements by blocking the sodium-potassium exchange through the use of non-steroidal anti-inflammatory drugs and potassium-sparing diuretics, such as spironolactone or amiloride.4
Etiologies of Hyperuricemia
| Underexcretion of uric acid | Overproduction of uric acid |
|---|---|
| Inherited: Gitelman syndrome, autosomal dominant tubulointerstitial kidney disease | Inherited monogenic defects in purine, sugar or adenosine triphosphate metabolism or other genetic abnormalities: Hypoxanthine-guanine phosophoribosyltransferase deficiency, PRP overactivity, glucose-6-phosphate dehydrogenase deficiency, Down syndrome |
| Renal: Insufficiency, lead nephropathy | Increased cell turnover: Hemolysis, lymphoproliferative disorder, myeloproliferative disorders, psoriasis |
| Cardiac: Congestive heart failure | Increased purine ingestion: Meat, seafood |
| Acidotic states: Ketoacidosis, lactic acidosis | Iatrogenic: Cytotoxic medication (contributing to tumor lysis syndrome) |
| Endocrinologic: Hyperparathyroidism, hypothyroidism | |
| Autoimmune: Sarcoidosis | |
| Iatrogenic: Cyclosporine, thiazide diuretics, loop diuretics, pyrazinamide, ethanol, laxatives |

