Thromboembolic events are major contributors to the morbidity and mortality of patients with giant cell arteritis (GCA), but little is known about how GCA may increase the risk of ischemic strokes. GCA-related stroke is described as an ischemic cerebral infarct occurring within three to four weeks of GCA diagnosis and treatment. It occurs in 3–7% of patients diagnosed with GCA and has a predilection for affecting the posterior circulation.
Many patients who experience stroke after GCA diagnosis may have risk factors for atherosclerotic disease. This makes it challenging to determine how GCA may have contributed to the patient’s stroke. The standard treatment for GCA-related stroke is high-dose glucocorticoids (i.e., 500–1,000 mg/day for three days). However, even when treatment is promptly administered, morbidity and mortality rates may be high.
The Case
A 72-year-old man presented to the emergency department for progressively worsening headaches, dizziness and jaw claudication. His erythrocyte sedimentation rate (ESR) was 97 mm/hr at that time, and he was diagnosed with GCA via temporal artery biopsy. He was treated with 60 mg of prednisone daily, which resolved his symptoms.
Three weeks later—while still taking prednisone at the 60 mg dose—he began feeling unwell, and his wife noted some changes in his speech. The patient awoke in the middle of the night to go to the bathroom and fell on his way back to bed due to left-sided weakness. He waited until morning—approximately six hours—before going into the emergency department.
In addition to the GCA diagnosis, he had a past medical history of type 2 diabetes mellitus, hypertension and hyperlipidemia.
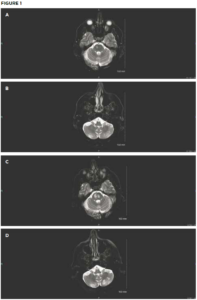
Initial brain MRI shows small areas of acute infarct in the right pons (A) and bilateral
cerebellum (B). Repeat brain MRI after sudden deterioration shows acute infarcts in the left pons with extension of infarcts in the right pons (C) and the left cerebellum (D). (Click to enlarge.)
On examination, the patient’s vital signs were within normal limits. He had severe upper and lower extremity weakness on his left side, along with left-sided facial droop and dysarthria. Laboratory testing revealed hyperglycemia of 396 mg/dL. His ESR was normal at 7 mm/hr. Throughout his emergency department stay, electrocardiograms and telemetry revealed no evidence of atrial fibrillation. Magnetic resonance imaging (MRI) showed an acute infarct in the right pons and bilateral inferior cerebellum (see Figures 1A and 1B). Magnetic resonance angiography (MRA) revealed a diminutive appearance of the vertebral arteries and the basilar artery, as well as severe bilateral siphon stenosis of the intracranial internal carotid arteries.
Because the patient was outside the window for thrombolytic therapy with tissue plasminogen activator, he was placed on dual antiplatelet therapy—aspirin and clopidogrel.
His condition initially improved, but four days after his initial presentation he awoke with an acute worsening of symptoms. He was treated with 1 g of methylprednisolone daily for three days, and his symptoms (e.g., facial droop, dysarthria, left-sided weakness) improved again. His treatment was complicated by hyperglycemia and steroid-induced psychosis.
Four days after his second episode, the patient had a third stroke. He was given 500 mg of methylprednisolone once and subcutaneous tocilizumab. A repeat MRI showed new acute infarcts in the left pons and extension of the right pons and left cerebellum infarcts (see Figures 1C and 1D).
Two days later, he had a fourth stroke, after which he was nonverbal and unable to respond to commands, and his left upper and lower extremity were flaccid. The patient was transferred for potential neurovascular intervention, but his condition continued to deteriorate, and he died shortly thereafter.
Discussion
GCA is a large-vessel vasculitis that occurs due to granulomatous inflammation and disruption of the internal elastic lamina that line large arteries. GCA is more common in women and people aged 70–80 years old than in men and younger populations. The most common presenting features are new-onset and worsening headaches, jaw claudication and scalp tenderness. Ischemic optic neuropathy is a known complication of GCA, occurring in approximately 7% of patients with GCA.1–4
Because patients with GCA often have concomitant risk factors for atherosclerosis, such as hypertension, hyperlipidemia and advanced age, as noted in our patient, diagnosing GCA-related stroke can be challenging. GCA-related strokes tend to favor the posterior circulation and are more likely to be bilateral than ischemic strokes not related to GCA. Pooling the results of three studies, the vertebrobasilar territory was affected in 73% (39 of 53) of GCA-related strokes.2–4
GCA rarely affects arteries that have passed the dura mater because these vessels do not contain the internal elastic lamina. Compared with atherosclerotic disease-related cerebrovascular accidents (CVAs), in patients with GCA, the middle cerebral artery is the most common site of CVAs (32–44%), and the combined vertebral and basilar territory is affected in approximately 40% of cases, with a slight preference for the basilar artery.5,6
A study focusing on the radiographic features of GCA-related stroke found that intracranial vasculitis most commonly affected the internal carotid arteries, followed by the vertebral artery and posterior cerebral artery, as was the case in the patient described above.7 For this reason, correlation between clinical and radiographic findings through an interdisciplinary team is necessary for prompt diagnosis. Even then, the prognosis for GCA-related stroke is poor because the majority of patients continue to experience disease progression.
Interventions
Glucocorticoids—The current standard treatment for GCA involves the timely administration of high-dose glucocorticoids. Recommendations are to start glucocorticoids when GCA is suspected, even prior to diagnosis confirmation with a temporal artery biopsy.8
When severe complications, such as vision loss, cerebral ischemia, cardiovascular ischemia or limb ischemia, are suspected, treatment with intravenous methylprednisolone at 500–1,000 mg/day for three days is recommended to reduce inflammation because glucocorticoids have good central nervous system (CNS) penetration and tend to have rapid onset.
For patients without severe complications, high-dose oral glucocorticoids are sufficient. Evidence also indicates that outcomes are better when tocilizumab is added.9
If a patient has a symptom relapse while being treated with intravenous glucocorticoids, the addition of a glucocorticoid-sparing agent, such as tocilizumab, is recommended. For patients with cranial ischemia, a glucocorticoid with higher mineralocorticoid activity may be added to reduce intracranial pressure.
Because high doses of glucocorticoids are used in GCA treatment, patients are more vulnerable to their side effects, such as hyperglycemia/diabetes mellitus, hypertension, fractures, gastrointestinal bleeding, sleeplessness, mood disturbances, cataracts, infections and psychosis. Relapse is a risk, even with normalized inflammatory markers, making it difficult for some patients to completely stop glucocorticoid therapy.10,11 Nevertheless, the benefit of glucocorticoids in the treatment of GCA likely outweighs the risk of complications when ischemia or vision loss are threatened.8
Cyclophosphamide—Tocilizumab is the most commonly used glucocorticoid-sparing agent for GCA. Another option is cyclophosphamide. An alkylating agent that inhibits protein synthesis by crosslinking DNA and RNA, cyclophosphamide is commonly used for malignant lymphomas and is approved by the U.S. Food & Drug Administration to treat breast cancer, disseminated neuroblastomas, retinoblastoma, minimal change nephrotic syndrome and ovarian adenocarcinomas.12 Because it’s a potent immune modulator, cyclophosphamide has been used in a variety of vasculitides, such as GCA.
Patients who are at high risk of, or have experienced, glucocorticoid-related adverse effects and have not had an appropriate response with other glucocorticoid-sparing treatments may benefit from treatment with cyclophosphamide. An observational study showed some efficacy of cyclophosphamide in 103 patients with GCA who had developed an intolerance to glucocorticoid treatment, relapsed or developed glucocorticoid dependency.13 Of these patients, 22% relapsed despite treatment and 78% responded to cyclophosphamide.13 Further of the 103 patients reviewed, 39% reported side effects with cyclophosphamide, including cytopenias and infections, with 12.6% of the patients discontinuing treatment due to these side effects.13
Tocilizumab—Another agent becoming more widely used to treat GCA is the interleukin (IL) 6 antagonist tocilizumab, with an improved side effect profile and decreased administration frequency. Tocilizumab is available as a once-weekly subcutaneous injection or a monthly intravenous infusion. The most common serious adverse events associated with its use include serious infections, gastrointestinal perforation, malignancy, myocardial infarction and cerebrovascular accidents.14 Although tocilizumab has only been studied in combination with initial prednisone therapy, it has demonstrated a higher rate of sustained remission than prednisone alone.9,15
Limitations of tocilizumab include cost, availability and limited data showing efficacy in treating intracranial GCA. The rarity of GCA-related stroke and the novelty of tocilizumab make it difficult to draw conclusions about the treatment’s effectiveness in preventing cerebral ischemic events. Interestingly, patients who suffered from ischemic complications due to GCA had lower tissue expression and serum levels of IL-6 than patients without ischemic events. A potential protective effect of IL-6 against ischemic events due to the angiogenic activity of IL-6 has been hypothesized.16
In monkey models, tocilizumab shows very poor CNS penetration, making its role in preventing intracranial ischemic events unclear.17 In a case study, a patient who switched from cyclophosphamide to tocilizumab experienced disease relapse resulting in GCA-related stroke and died shortly thereafter.18
Antiplatelet therapy—Due to significant overlap in GCA and atherosclerotic disease, it has been postulated that patients with GCA and atherosclerotic risk factors may benefit from antiplatelet or anticoagulant therapy at the time of diagnosis.
The role of aspirin has generated debate over the years, with the ACR recommending aspirin use when a patient has additional antiplatelet requirements. This approach is supported by a small-scale study involving 143 patients that found a decreased incidence of ischemic events, from 48% to 16.2%, in patients receiving antiplatelet or anticoagulant therapy.19
Given the low cost and availability of aspirin and statins, it may be beneficial to start patients on antiplatelet and/or statin therapy to prevent GCA-related stroke. This approach may be especially effective if the patient has pre-existing risk factors, such as a history of smoking, hyperlipidemia, hyperglycemia/diabetes mellitus and family history of atherosclerotic disease.
Conclusion
The prognosis for patients with GCA-related stroke remains poor, and the rate of relapse is high. Patients with concomitant atherosclerotic risk factors at the time of GCA diagnosis may benefit from antiplatelet and statin therapy, but further research is needed.
Patient education, multidisciplinary engagement between rheumatologists, neurologists and radiologists, and prompt intervention when GCA-related stroke is identified may help improve patient outcomes.
 Jessica Meek, MD, is an internal medicine resident at Brown University/Kent Hospital, Providence, R.I. She is interested in all things internal medicine and loves to teach.
Jessica Meek, MD, is an internal medicine resident at Brown University/Kent Hospital, Providence, R.I. She is interested in all things internal medicine and loves to teach.
 Anna Coppinger, DO, is an internal medicine resident at Brown University/Kent Hospital. She has a passion for patients with chronic medical conditions and autoimmune disorders.
Anna Coppinger, DO, is an internal medicine resident at Brown University/Kent Hospital. She has a passion for patients with chronic medical conditions and autoimmune disorders.
 Sejal Khan, DO MS, is an internal medicine resident at Brown University/Kent Hospital. Driven by a passion for delivering exceptional patient care, he is interested in innovative solutions in the field of musculoskeletal medicine, particularly the application of ultrasound for the treatment of musculoskeletal conditions.
Sejal Khan, DO MS, is an internal medicine resident at Brown University/Kent Hospital. Driven by a passion for delivering exceptional patient care, he is interested in innovative solutions in the field of musculoskeletal medicine, particularly the application of ultrasound for the treatment of musculoskeletal conditions.
References
- Chen JJ, Leavitt JA, Fang C, et al. Evaluating the incidence of arteritic ischemic optic neuropathy and other causes of vision loss from giant cell arteritis. Ophthalmology. 2016 Sep;123(9):1999–2003.
- Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy proven giant cell arteritis. Medicine (Baltimore). 2009 Jul;88(4):227–235.
- Samson M, Jacquin A, Audia S, et al. Stroke associated with giant cell arteritis: A population-based study. J Neurol Neurosurg Psychiatry. 2015 Feb;86(2):216–221.
- de Boysson H, Liozon E, Larivière D, et al. Giant cell arteritis–related stroke: A retrospective multicenter case-control study. J Rheumatol. 2017 Mar;44(3):297–303.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011 Sep;365(11):993–1003.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 Mar;352(13):1305–1316.
- Sanchez-Alvarez C, Hawkins AS, Koster MJ, et al. Clinical and radiographic features of giant cell arteritis with intracranial involvement. ACR Open Rheumatol. 2020 Aug;2(8):471–477.
- Maz M, Chung SA, Abril A, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of giant cell arteritis and Takayasu arteritis. Arthritis Care Res. 2021 Aug;73(8):1071–1087.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017 Jul;377(4):317–328.
- Kermani TA, Warrington KJ, Cuthbertson D, et al. Disease relapses among patients with giant cell arteritis: A prospective, longitudinal cohort study. J Rheumatol. 2015;42(7):1213–1217.
- Proven A, Gabriel SE, Orces C, et al. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Care Res. 2003 Oct;49(5):703–708.
- Ogino MH, Tadi P. Cyclophosphamide. In: StatPearls. Treasure Island, Fla.: StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK553087.
- de Boysson H, Boutemy J, Creveuil C, et al. Is there a place for cyclophosphamide in the treatment of giant-cell arteritis? A case series and systematic review. Semin Arthritis Rheum. 2013 Aug;43(1):105–112.
- Schiff MH, Kremer JM, Jahreis A, et al. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141.
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2016 May; 387(10031):1921–1927.
- Hernández-Rodríguez J, Segarra M, Vilardell C, et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant cell arteritis: Angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation. 2003 May;107(19):2428–2434.
- Nellan A, McCully CML, Cruz Garcia R, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018 Aug;132(6):662–666.
- Cox B, Fulgham J, Klaas J. Recurrent stroke in giant cell arteritis despite immunotherapy. Neurologist. 2019 Jul; 24(4):139–141.
- Lee MS, Smith SD, Galor A, et al. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 2006 Oct; 54(10):3306–3309.