Alila Medical Media / shutterstock.com
Behçet’s disease is a chronic, relapsing and remitting vasculitis with multisystem involvement. Commonly referred to as the Silk Road disease due to its prevalence in the Asian and Mediterranean region of the traditional Silk Road, Behçet’s was first described by Hippocrates as a triad of symptoms—genital and oral ulcers with uveitis—and attributed to links with other illnesses. The Turkish physician Hulusi Behçet was the first to distinguish the freestanding symptoms as a disease manifestation of their own and came to describe Behçet’s disease. Behçet’s can affect arteries and veins of all sizes. When it involves the central nervous system and intestines, it results in a poorer prognosis. Current genetic and environmental links to the disease are being evaluated, including upregulation of HLA-B27 and heat shock protein 60 as well as association with herpes simplex virus and streptococcal virus.
The Case
A 34-year-old woman presented with a chief complaint of recurrent abdominal pain. She had a past medical history of Behçet’s disease with multiple abdominal symptoms and gastrointestinal involvement. Her past surgical history included an appendectomy and cholecystectomy. An abdominal X-ray was negative. Because of the patient’s history, she was admitted for abdominal pain control secondary to a Behçet’s flare.
She was started on a hydromorphone patient-controlled analgesia (PCA) along with continuing her home steroids and azathioprine. She was started on standard heparin subcutaneous injections for deep vein thrombosis prophylaxis while an inpatient. Three days into her hospital stay, the patient syncopized in the bathroom.
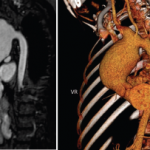
Vitals at the time showed a blood pressure of 70/40 with sinus rhythm. A computed tomography (CT) scan of the head was negative for an acute bleed. Her troponins continued to rise, peaking at 1.68. A transthoracic echocardiogram showed reduced RV systolic function and RA and RV dilation. Her D-dimer was elevated. A CT angiogram of the chest showed bilateral segmental pulmonary emboli (see Figures 1 and 2). Lower extremity deep vein thrombosis studies were negative.
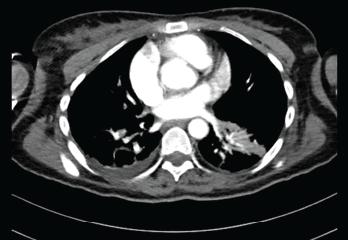
The Pulmonary Embolism Response Team was activated and suggested catheter-directed tissue plasminogen activator (tPA), which the patient refused. She was then started on a heparin drip and transferred to the intensive care unit for closer monitoring. Her hypercoagulable workup results were negative. The patient was bridged to rivaroxaban, and immunosuppressive agents were continued, with significant improvement in clinical status.
Discussion
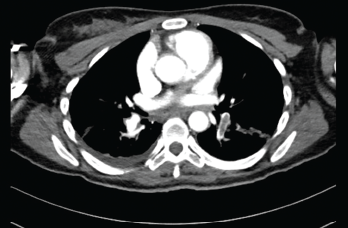
Submassive pulmonary embolism. Filling defects seen in the pulmonary artery.
This case highlights an unprovoked massive pulmonary embolism in a young patient with Behçet’s disease. A paucity of literature addresses pulmonary manifestations of Behçet’s, and the prognosis, morbidity and mortality involved with this patient population.
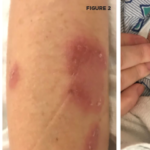
Behçet’s is an autoimmune disease commonly presenting with recurrent oral and genital ulcers, as well as skin and eye involvement. Because Behçet’s does not have pathognomonic findings limited only to its own disease process, the diagnosis is made using criteria established by the International Study Group for Behçet’s Disease in 1990. The criteria emphasize recurrent oral and genital ulcers, as well as eye lesions, skin lesions and a positive pathergy test. A positive pathergy test stands as a unique disease feature, showcasing immune system hyper-reactivity and signals, and upregulated inflammatory response. Patients are typically in the second to third decade of life at onset with an equal male to female predominance. Younger age of onset and male sex are markers of more severe disease.
The etiology of this disease is not well understood, and vascular involvement is thought to be due to inflammation localized to the vasa vasorum. Neutrophil activation and migration in conjunction with free radical generation have been proposed as the underlying mechanism. More recent studies suggest a role of T lymphocyte upregulated immune response as an important part of disease pathogenesis. Clinically active disease has been associated with oligoclonal T cell expansion. An increased T cell response has been shown against peptides derived from heat shock proteins, leading to the theory that heat shock proteins are activating antigens in Behçet’s. Streptococcal and herpes simplex virus have also induced a T cell proliferative response, further complicating the underlying etiology with an antimicrobial theory.
Vascular involvement occurs in up to 40% of patients with a male predisposition. Thrombosis is usually secondary to underlying vasculitis, with lower extremity venous thromboembolism presenting as the most common vascular abnormality in patients with Behçet’s. Multiple studies have tried to establish a genetic link between the development of BD and HLA-B51 positivity; however, results have thus far been controversial. In Turkey, the risk of disease development with this allele is 13.1% compared to 1.3% in the U.S. It has been proposed that individuals with HLA-B51 positivity tend to run a more refractory symptom course; however, this has been only been observational.
The pulmonary manifestations of this disease are uncommon. The pulmonary arteries are the second most common area of arterial involvement, with the aorta being first. Arterial aneurysms, venous thrombosis, pulmonary infarction, pulmonary vessel inflammation and reactivity and organizing pneumonias are known pulmonary complications. The true prevalence of pulmonary involvement in Behçet’s is unknown.
Pulmonary artery involvement has a prevalence rate of less than 5%, presenting as pulmonary artery aneurysm and less likely as pulmonary artery thrombosis. Pulmonary artery aneurysms present with hemoptysis, and rupture can lead to flare up of an acute vasculitis, with resulting in situ pulmonary thrombosis. Pulmonary artery embolism is considered one of the most severe and worst prognostic manifestations of the disease. In most patients, pulmonary artery embolism is isolated and does not occur in concordance with lower extremity venous thrombosis. Although deep venous thrombosis can occur, pulmonary embolisms from a distal source are uncommon as the clot is adherent to the inflamed endothelial lining of vessels.
Hyperhomocysteinemia has also been proposed as an independent risk factor for development of pulmonary thrombosis in Behçet’s. Elevated levels of homocysteine have been shown to result in an increased predisposition to arterial and venous thrombosis. The mechanism involves damage to endothelial cells due to production of hydroxyl radicals from excess homocysteine. It may also promote the proliferation of vascular lining and may play a role in degrading innate anti-thrombotic measures. A meta-analysis in 2010 that looked at 16 studies with a total of 979 patients and concluded homocysteine levels were higher in Behçet’s patients with thrombosis compared with those without.
Adequate treatment for pulmonary thrombosis includes anticoagulation in conjunction with immunosuppressive medications. Anti-inflammatory medication is the mainstay of Behçet’s disease treatment. In 2012, a retrospective cohort of 807 patients with Behçet’s and venous thrombosis showed that immunosuppressive agents significantly reduced the risk of venous thrombosis relapse rate—especially the use of glucocorticoids. Further studies are needed to assess the longevity of anticoagulation for thrombotic patients in Behçet’s; however, immunosuppression is usually lifelong.
Conclusion
This patient presentation shows the importance of possessing high clinical suspicion of vascular involvement in patients with Behçet’s disease presenting with new-onset fever, dyspnea, hemoptysis or syncope. It also hopes to showcase the complex clinical management of these patients and the ongoing challenge to prevent recurrent vascular complications.
 Aiza Tariq, MD, is currently a PGY-2 internal medicine resident in New York City. She is interested in rheumatic diseases and in pursuing a career in rheumatology. Clinically, her research has included hypercoagulable and autoinflammatory diseases.
Aiza Tariq, MD, is currently a PGY-2 internal medicine resident in New York City. She is interested in rheumatic diseases and in pursuing a career in rheumatology. Clinically, her research has included hypercoagulable and autoinflammatory diseases.
 Jasim Alidina, MD, is currently a PGY-3 resident in radiology. His clinical interests lie in advanced musculoskeletal imaging. He enjoys collaborating with rheumatology on complex and challenging cases.
Jasim Alidina, MD, is currently a PGY-3 resident in radiology. His clinical interests lie in advanced musculoskeletal imaging. He enjoys collaborating with rheumatology on complex and challenging cases.
References
- Suzuki Kurokawa M, Suzuki N. Behçet’s disease. Clin Exp Med. 2004 Sep;4(1):10–20.
- Desbois AC, Wechsler B, Cluzel P, et al. [Cardiovascular involvement in Behçet’s disease.] Rev Med Interne. 2014 Feb;35(2):103–111.
- Calamia K, Schrimer M, Melikogluc M. Major vessel involvement in Behçet’s Disease: An update. Curr Opin Rheumatol. 2011 Jan;23(1):24–31.
- Seyahi E, Yurdakul S. Behçet’s syndrome and thrombosis. Mediterr J Hematol Infect Dis. 2011;3(1):e2011026.
- Desbois AC, Weschler B, Resche-Rigon M, et al. Immunosuppressants reduce venous thrombosis relapse in Behçet’s disease. Arthritis Rheum. 2012 Aug;64(8):2753–2760.
- La Regina M, Orlandini F, Prisco D, et al. Homocysteine in vascular Behçet disease: A meta-analysis. Arterioscler Thromb Vasc Biol. 2010 Oct;30(10):2067–2074.
- Erkan F, Gül A, Tasali E. Pulmonary manifestations of Behçet’s disease. Thorax. 2001 Jul;56(7):572–578.