Patients with chronic kidney disease (CKD) often experience joint pain due to various etiologies, including crystalline arthropathies, renal osteodystrophy, amyloid arthropathy, erosive osteoarthritis, avascular necrosis and even erosive spondylarthrosis.1 Below, we present a case of crystalline arthropathy in a patient with chronic kidney disease, mistaken for gout.
The Case
A 29-year-old man was admitted to the hospital with bacteremia. He had a complex medical history, which included an orthotopic liver transplant at three years old, complicated by chronic rejection and tacrolimus-associated end-stage renal failure on hemodialysis for the past two years. During his admission, he developed acute, chronic pain in his hands, elbows and ankles. He localized the pain in his hands to proximal interphalangeal joints (PIPs) and distal interphalangeal joints (DIPs), bilaterally. He denied joint redness or warmth, morning stiffness, back pain, rash or ulcers. He had fever associated with bacteremia, which resolved with antibiotic therapy. He reported similar symptoms in his hands in the past, and had been treated for presumed gout with febuxostat. His other medications included low-dose prednisone, amlodipine, lisinopril, mycophenolate mofetil, omeprazole, sevelamer, tacrolimus, ursodiol and potassium chloride. He was not taking any vitamin C supplementation. Family history was notable for no known history of chronic kidney disease or autoimmune disease.
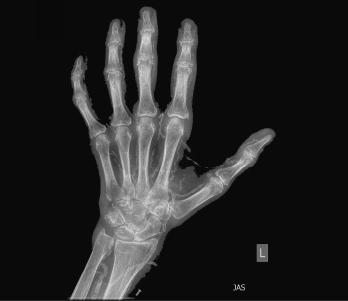
Figure 1: An X-ray of the left hand showed extensive vascular and periarticular calcification.
Physical examination revealed slightly swollen and tender PIPs. He had contractures of both elbows and pain with movement in all directions, as well as decreased range of motion in his ankles without effusion. Ultrasound of his PIP joints, ankles and the first metatarsal phalangeal joints of his feet did not reveal evidence of uric acid crystal deposition, double contour sign within the joints or tendons, or joint effusion amenable to aspiration. Radiographs revealed dense arterial calcifications in his hands and feet bilaterally, scattered juxta-articular soft tissue calcifications and no radiographic findings of gout (see Figures 1 and 2).
His uric acid (UA) prior to urate-lowering therapy was only 4.4 mg/dL (normal range: 3.6–8.5 mg/dL); after initiation of febuxostat and dialysis, his UA measured 2.4 mg/dL. He tested negative for rheumatoid factor and anti-CCP antibodies. Given the presence of soft tissue and vascular calcifications, additional laboratory test were conducted, including parathyroid hormone (42 pg/mL), calcium (9.3 mg/dL) and phosphorus (3.6 mg/dL), all of which were within normal limits. A plasma oxalate level was highly elevated at 69.1 mcmol/L (normal range: <1.6 mcmol/L), raising concern for the diagnosis of oxalic acid arthropathy. His daily dose of prednisone was increased to 30 mg (which improved his joint pain and swelling) with the goal of renal transplant in the future.
Discussion
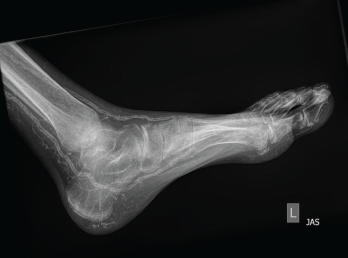
Figure 2: An X-ray of the left foot showed extensive vascular calcifications and no radiographic evidence of gout.
Crystalline arthropathies, such as gout, calcium pyrophosphate deposition (CPPD) disease and hydroxyapatite deposition, are not uncommon in patients with end-stage renal disease. The gold standard for crystalline diagnosis remains synovial fluid aspiration demonstrating the specific crystal, but in this case there was no joint effusion present for aspiration. The presence of monosodium urate (MSU) or CPPD crystals can be seen under polarized microscopy upon routine synovial fluid analysis, but other calcium crystals (e.g., hydroxyapatite and oxalate) are not typically reported in standard laboratory synovial fluid analysis and require additional stains (e.g., alizarin red) to distinguish crystalline etiology. Without confirmation of synovial fluid analysis, these crystal arthropathies can prove difficult to clinically distinguish from one another.
Gout is incredibly common in end-stage renal disease and is often easier to identify earlier in the course of the disease when it involves the first metatarsophalangeal joint. In renal failure, and as gout progresses, it can present as polyarticular and can be symmetric, involving the midfoot, ankle, knee and elbow.2
CPPD disease, often considered a gout mimic, has multiple presentations, from acute CPPD arthritis (similar to gout attacks) to osteoarthritis with CPPD to chronic inflammatory arthritis (i.e., a pseudo-rheumatoid arthritis presentation). It can coexist with gout, septic arthritis, osteoarthritis, rheumatoid arthritis and oxalate arthropathy.3,4
Oxalic acid arthropathy is an infrequent arthritic manifestation of hyperoxaluria that is associated with calcium oxalate deposition in tendons, cartilage, synovium and even blood vessels, as in our patient.5 Oxalate is primarily excreted through the kidney, and therefore hyperoxaluria can be the result of decreased excretion from renal failure. vitamin C supplementation can exacerbate it.6
Renal oxalosis occurs in a large majority of patients requiring long-term hemodialysis, and concentrations as high as 30 mcmol/L are not uncommon.3 The resulting inflammation can manifest as a symmetric polyarthritis that typically involves the PIP, metacarpophalangeal joints, knee, elbow and ankle joints.3 Deposition in blood vessels can further confound the picture because it can mimic vasculitis when severe.5
Important: The gold standard for oxalic acid arthropathy diagnosis is demonstration of positively birefringent crystals within synovial fluid or tissue that stain positive with alizarin red.
Imaging can help crystalline disease diagnosis when synovial fluid is not available for aspiration. The ACR/EULAR classification criteria for gout include use of imaging with ultrasound or dual-energy computed tomography (DECT) to identify the presence or absence of a double contour sign on ultrasound or urate deposition on DECT, and/or radiographic evidence of gout-related joint damage (e.g., gout-related erosion with a cortical break with a sclerotic margin and overhanging edge, excluding the gull wing appearance typically seen in erosive osteoarthritis).7
Ultrasound and CT also can demonstrate chondrocalcinosis, the deposition of CPPD in hyaline and fibrocartilage. The presence of chondrocalcinosis, however, can be an incidental finding and does not provide definitive proof that CPPD is causing the joint pain.7,8
No data exists on use of ultrasound or DECT to distinguish oxalate arthropathy. There is research interest on altering current DECT algorithms to try to differentiate calcium hydroxyapatite from CPPD. Radiographs of joints affected by these less common crystal arthropathies often have nonspecific radiographic findings such as vascular calcifications, which can also appear in renal osteodystrophy and chronic kidney disease and therefore do not confirm the diagnosis.9 This overlap of associated radiographic findings makes it difficult to determine if a finding is related to the primary pathology causing a patient’s symptoms, or if it is incidental and without clinical correlation.
In our case, the patient’s oxalate level was severely elevated—in a range more commonly seen in primary hereditary oxalosis and in which organ and joint involvement is almost universal.3,10 Given that there was no effusion to aspirate and that a tissue biopsy would not change management, these interventions were deferred.
Without crystal-proven fluid/tissue analysis, it’s difficult to say for certain his symptoms were solely attributed to hyperoxaluria because CPPD, hydroxyapatite arthropathy and renal osteodystrophy can all contribute to calcium deposition and arthropathy. However, the pattern of DIP joint involvement is less common with hydroxyapatite arthropathy or renal dystrophy, and CPPD would not explain the vascular calcifications. The lack of MSU and the appearance of CPPD crystals on ultrasound also lowered our suspicion for gout and CPPD.
The most effective management for oxalate arthropathy is preventive, with the removal of oxalate through hemodialysis and limiting ascorbic acid intake. However, even with hemodialysis, removal is incomplete and often requires high flux membranes or daily hemodialysis.3 Systemic or intra-articular steroids remain the mainstay therapy for acute oxalate arthritis in patients with renal failure. In the absence of renal dysfunction, such as in hereditary or primary hyperoxaluria, non-steroidal anti-inflammatory drugs and colchicine can be used.
Data exist indicating the inflammatory pathways in oxalic acid arthropathic are interleukin 1β mediated, suggesting
IL-1 inhibition may be another option in the future.11
 Katherine Yates, MD, completed her medical degree at Harvard Medical School and is an internal medicine resident at Vanderbilt University Medical Center, Nashville, Tenn. She is considering pursuing a rheumatology fellowship.
Katherine Yates, MD, completed her medical degree at Harvard Medical School and is an internal medicine resident at Vanderbilt University Medical Center, Nashville, Tenn. She is considering pursuing a rheumatology fellowship.
 Erin H. Penn, MD, received her medical degree from Jefferson Medical College in Philadelphia, completed her residency in internal medicine at Brigham and Women’s Hospital in Boston and is currently a fellow at Brigham and Women’s Hospital in rheumatology. Her areas of interest within rheumatology include nutritional, dermatologic and pulmonary-vascular manifestations of systemic rheumatologic disease. As a fellow, she is investigating the relationship between nutritional deficiencies and wellness in autoimmune disease, and associations between dermatomyositis and interstitial lung disease, while also training to be a clinician educator.
Erin H. Penn, MD, received her medical degree from Jefferson Medical College in Philadelphia, completed her residency in internal medicine at Brigham and Women’s Hospital in Boston and is currently a fellow at Brigham and Women’s Hospital in rheumatology. Her areas of interest within rheumatology include nutritional, dermatologic and pulmonary-vascular manifestations of systemic rheumatologic disease. As a fellow, she is investigating the relationship between nutritional deficiencies and wellness in autoimmune disease, and associations between dermatomyositis and interstitial lung disease, while also training to be a clinician educator.
 Minna J. Kohler, MD, is the director of the Rheumatology Musculoskeletal Ultrasound (MSKUS) program at Massachusetts General Hospital in Boston and an instructor in medicine at Harvard Medical School. Dr. Kohler has educational and research interests in point-of-care ultrasound and innovative imaging related to arthritis, crystalline disease, vasculitis and other musculoskeletal and rheumatic diseases. She provides specialized clinical care as part of the Massachusetts General Hospital Gout and Crystal Arthropathy Center.
Minna J. Kohler, MD, is the director of the Rheumatology Musculoskeletal Ultrasound (MSKUS) program at Massachusetts General Hospital in Boston and an instructor in medicine at Harvard Medical School. Dr. Kohler has educational and research interests in point-of-care ultrasound and innovative imaging related to arthritis, crystalline disease, vasculitis and other musculoskeletal and rheumatic diseases. She provides specialized clinical care as part of the Massachusetts General Hospital Gout and Crystal Arthropathy Center.
Takeaways
- Patients with renal failure are at risk for many forms of arthritis that can sometimes be clinically indistinguishable.
- Clinicians should have a high index of suspicion for oxalate arthropathy in patients with long-standing renal failure or who are on hemodialysis. Consider oxalate levels and obtain synovial biopsy with alizarin red staining to confirm the diagnosis.
- Although gout is a common crystalline arthropathy associated with chronic kidney disease, imaging with clinical correlation can help distinguish crystal etiology when joint effusion/tissue is not available for analysis. Oxalic acid arthropathy is not necessarily distinguishable on imaging alone.
- Determining high levels of oxalate can help promote modification of hemodialysis regimens and avoid inappropriately treating patients for other types of arthritis, such as gout.
References
- Bardin T, Richette P. Rheumatic manifestations of renal disease. Curr Opin Rheumatol. 2009 Jan;21(1):55–61.
- Dalbeth N, Stamp L, Merriman T. (2016) Gout. Oxford: Oxford University Press.
- Lorenz EC, Michet CJ, Milliner DS, et al. Update on Oxalate Crystal Disease. Curr Rheumatol Rep. 2013 Jul;15(7).340.
- Terkeltaub R. Gout and Other Crystal Arthropathies. Philadelphia: Elsevier Saunders, 2012.Reginato AJ, Seoane JLF, Alvarez CB, et al. Arthropathy and cutaneous calcinosis in hemodialysis oxalosis. Arthritis Rheum. 1986 Nov;29(11):1387–1396.
- Rathi, S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: A case report. J Med Case Rep. 2007 Nov 27;1:155.
- Naredo E, Lagnocoo A. One year in review 2017: Ultrasound in crystal arthritis. Clin Exp Rheumatol. 2017 May–Jun;35(3):362–367.
- Choi MH, Mackenzie JD, Dalinka MK. Imaging features of crystal-induced arthropathy. Rheum Dis Clin North Am. 2006 May;32(2):427–446.
- Jablonski KL, Chonchol M. Vascular calcification in end-stage renal disease. Hemodial Int. 2013 Oct;17 Suppl 1:S17–21.
- Milliner DS, Eickholt JT, Bergstralh EJ, et al. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994 Dec 8;331(23):1553–1558.
- Mulay SR, Kulkarni OP, Rupanagudi KV, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest. 2013 Jan;123(1):236–246.