Figure 1: Computed Tomography VGstockstudio / shutterstock.com
Pulmonary nodules are common; most are benign, but the differential diagnosis is broad and includes life-threatening possibilities.1 Our patient is a former smoker who has a history of a complex autoimmune disease and multiple pulmonary nodules. This case was challenging, but clinical, radiographic and histologic clues helped lead to the correct diagnosis.
Case Presentation
The patient is a 74-year-old man with a past medical history remarkable for hypertension, hyperlipidemia, gastroesophageal reflux disease, emphysema, asbestos exposure and seropositive (rheumatoid factor and anti-cyclic citrullinated protein antibody) rheumatoid arthritis/scleroderma/systemic lupus erythematosus (SLE) overlap syndrome. He has a 100 pack-year smoking history, although he quit smoking 22 years ago.
The patient was diagnosed with seropositive rheumatoid arthritis 15 years ago. His initial treatment was 15 mg of oral methotrexate weekly and 40 mg of subcutaneous adalimumab every 14 days. His methotrexate was tapered to 10 mg weekly, and the patient did well.
His disease course was complicated by the development of bulky rheumatoid nodules on the extensor surfaces of his elbows. After surgical removal of the nodules, he was taken off methotrexate and switched to 1 g of sulfasalazine twice daily to reduce the risk of rheumatoid nodulosis. Due to his moderately active disease, his therapy was changed from sulfasalazine to 20 mg of leflunomide daily.
The patient stopped taking adalimumab because he felt well.
The pulmonary nodules in rheumatoid pneumoconiosis develop cavitations or calcifications in 10% of cases.
Four years ago, the patient complained of Raynaud’s phenomenon. A physical examination revealed skin tightness from his fingertips to his proximal interphalangeal joints.
Serologic tests were positive for anti-nuclear antibody (ANA; 1:160, in a speckled pattern) and anti-centromere antibody (ACA; 2.8 AI; reference range [RR]: 0.0–0.9 AI). Tests for anti-SSA/Ro antibody, anti-SSB/La antibody, anti-nuclear ribonucleoprotein (anti-RNP) antibody, anti-Smith antibody and anti-topoisomerase I antibody were negative.
Two years ago, the patient had a flare of inflammatory arthritis, and treatment was started with 125 mg of subcutaneous abatacept weekly.
A few months later, the patient developed dyspnea due to serositis. He had no fever, cough, weight loss, night sweats or joint pain. A QuantiFERON gold test was negative for tuberculosis.
Results of pleural fluid studies included: albumin of 3.1 g/dL (RR: 3.5–5.0 g/dL), white blood cell count of 2535/µL (RR: 4,000–11,000/µL) with 44% neutrophils, glucose of 2 mg/dL (RR: 60–99 mg/dL) and a lactate dehydrogenase of 2,308 U/L (RR: 140–280 U/L).
Cultures for bacteria, fungi and acid-fast bacilli showed no growth. Cytology of the fluid revealed no malignant cells. The pleural fluid studies were consistent with an exudative, rheumatoid pleural effusion.
The patient experienced atypical chest pain. Evaluation for acute coronary syndrome was negative.
One month later, chest imaging showed a pericardial effusion and a repeat echocardiogram was consistent with tamponade. A pericardial window was performed to manage cardiac tamponade, and biopsy of the pericardium revealed fibrous tissue with congestion, chronic inflammation and granulation tissue.
Cultures of the pericardial fluid for bacteria, acid-fast bacilli and fungi were unrevealing. Anti-proteinase-3 (PR3) antibody was 4.3 U/mL (RR: 0–3.5 U/mL). The patient also had reduced C4 complement, 6.9 mg/dL (RR: 9–36 mg/dL), and IgM anti-cardiolipin antibody was mildly elevated at 39 U/mL (RR: 1–12 U/mL). The patient had no joint inflammation or renal disease at that time.
The patient had a history of urticaria after taking non-steroidal anti-inflammatory drugs; therefore, he was treated with 10 mg of prednisone daily and 0.6 mg of colchicine twice daily. Sulfasalazine was discontinued because serositis was reported as a possible side effect of this drug in case reports, and 200 mg of hydroxychloroquine twice daily was initiated.
The following tests were performed: anti-double-stranded DNA (anti-dsDNA) antibody <1 IU/mL (RR: 0–9 IU/mL), anti-RNP antibodies <0.2 AI (RR: 0.0–0.9 AI), anti-nuclear cytoplasmic antibodies <1:20 (RR: <1:20 titer), anti-myeloperoxidase antibody <9.0 U/mL (RR: 0.0–9.0 U/mL) and anti-proteinase-3 antibody <3.5 U/mL (RR: 0.0–3.5 U/mL).
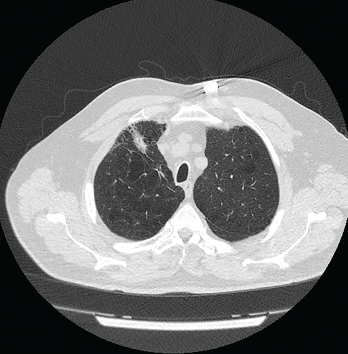
Pulmonary function studies were consistent with moderate obstructive lung disease and moderate decrease in diffusion capacity. Computed tomography (CT) scan of the thorax revealed severe emphysematous changes, a 1.8 cm x 1.6 cm pleural-based nodule in the anterior aspect of the right upper lobe and multiple sub-centimeter pulmonary nodules (see Figure 1).
Two percutaneous transthoracic biopsies of the largest left upper lobe and right upper lobe nodules showed patchy interstitial fibrosis and anthracotic pigmented macrophages. No malignancy or granuloma was identified on histology, and cultures for bacteria were negative.