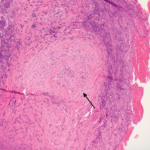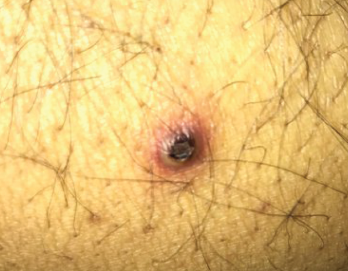
Figure 1. The patient had nodular pus-filled cystic lesions with violaceous borders on his left ear, right cheek, fingers and legs.
A 42-year-old man with a history of ulcerative colitis (UC), primary sclerosing cholangitis (PSC) and chronic sinusitis was referred to a rheumatologist to evaluate for a possible diagnosis of systemic vasculitis. This patient had developed new skin lesions, gingival hypertrophy and ulcerating tracheobronchitis, concerning for possible granulomatosis with polyangiitis (GPA).
Since 1994, the patient had sinusitis with occasional bloody discharge and nasal turbinate hypertrophy demonstrated on imaging. He was followed by an otolaryngologist, and his symptoms were well controlled with budesonide nasal rinses. In 1996, the patient presented to the hospital with abdominal pain and bloody diarrhea. A subsequent colonoscopy and biopsy confirmed a diagnosis of UC. He was treated with mesalazine, and in 1998, when he developed PSC, ursodiol was added.
Overall, his UC was well controlled until 2012. At that time, he began requiring intermittent steroids for UC flares, and in 2015, his gastroenterologist started treatment with vedolizumab.
In late 2016, the patient developed nodular, pus-filled cystic lesions with violaceous borders on his left ear, right cheek, fingers and legs (see Figure 1). Biopsy of these cutaneous manifestations were suggestive of neutrophilic folliculitis, a variant of pyoderma gangrenosum.1
Around the same time, the patient also started having severely painful, bleeding gingival hypertrophy (see Figure 2). Based on the appearance of his gums, this symptom was thought to be strawberry gingivitis, a rare, but classic, feature of GPA.2 Consequently, the possibility of an overlapping vasculitis concurrently occurring with the patient’s inflammatory bowel disease (GPA-IBD overlap) was suggested. As a result, azathioprine and prednisone were added to the patient’s medication regimen.
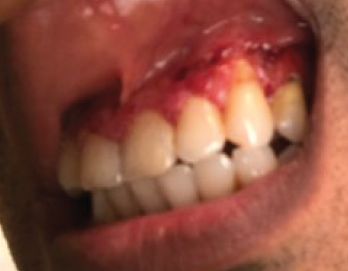
Figure 2. The patient presented with severe, painful, bleeding gingival hypertrophy.
After review with multiple specialists, gingival biopsies in January 2017 demonstrated pyostomatitis vegetans, which is a known extra-intestinal manifestation of IBD.
Sinus biopsies were also performed and did not show any evidence of vasculitis. Further, serologic evaluation during this period of diagnostic inquiry was variable. In March 2016, the patient was positive for C-ANCA (anti-neutrophilic cytoplasmic antibodies), but was PR3-ANCA and MPO-ANCA negative. In February 2017, he had an atypical P-ANCA (perinuclear ANCA) with a PR3-ANCA of 49.6 (normal <20). In March 2017, all ANCA serologies were negative.
Over the next eight months, the patient was treated with increasing doses of azathioprine and prednisone, up to 150 mg once daily and 50 mg once daily, respectively. But his pyostomatis vegetans failed to respond, and he continued to have significantly distressing gum pain and bleeding.
In September 2017, in an attempt to control this symptom, he was switched from vedolizumab to infliximab. This change initially improved his gingival manifestations after the first dose; however, subsequent doses, including dose escalations, did not achieve the same therapeutic effect. Additionally, his previously well-controlled gastrointestinal symptoms flared, and the decision was made to switch him back to vedolizumab.
In March 2018, the patient was admitted to the hospital with decompensated liver cirrhosis and an acute portal vein thrombosis. He underwent liver transplant and was discharged home.
In managing IBD, it is vital to work closely with other specialists to determine the true etiology of symptoms. Gastroenterologists, in particular, play an important role, ensuring bowel disease activity is suppressed prior to considering overlap syndromes.
One month later, he developed daily fevers and hemoptysis, again requiring hospital admission. A pulmonologist was consulted, and bronchoscopy revealed extensive ulcerative tracheobronchitis. At the same time, a colonoscopy demonstrated active inflammation in the rectum and sigmoid. His pulmonary presentation was, therefore, deemed to be yet another rare extra-intestinal manifestation of his UC and part of his current flare. For management, he was given high-dose steroids, ustekinumab and mesalamine. This therapy stabilized his symptoms, and he was discharged in May 2018 with a tapering dose of prednisone.
In July 2018, the patient passed away due to complications from his liver transplant.
Various specialists from multiple disciplines had been involved in managing his disease. Despite several sinus, gum, colonic and skin biopsies, no histological evidence of vasculitis was ever revealed. Moreover, colonic biopsies and visual inspection on colonoscopy were in keeping with active UC. Therefore, the consensus was that his presentation was in keeping with IBD and rare extra-intestinal manifestations rather than a case of GPA-IBD overlap.
Discussion
The diagnosis of true GPA-IBD overlap is challenging given the rarity of this condition. To the best of our knowledge, fewer than 35 cases have been reported in the literature.3-7 In general, patients with GPA-IBD overlap are women.3 Although vasculitis can precede or concurrently present with IBD, most patients tend to develop bowel symptoms first.3-7 The vasculitic manifestations in GPA-IBD overlap are variable, and reports of renal, neurological, otolaryngologic and cutaneous symptoms have been made.3-7 In addition, almost all patients invariably have some degree of pulmonary involvement, although the most common symptoms are non-specific, such as cough, dyspnea and fever.5 Further, most previous reported cases of GPA-IBD overlap have involved positive ANCA serology and demonstrated histological confirmation of both IBD and vasculitis.3-7
The diagnosis of GPA-IBD overlap is further complicated because certain extra-intestinal manifestations of IBD can mimic GPA, as seen in our case. Extra-intestinal manifestations occur in up to 30% of IBD patients and are generally more common in UC than in Crohn’s disease.8,9 Up to 15% of IBD patients develop cutaneous manifestations, such as pyoderma gangrenosum.1
Pyoderma gangrenosum is classically described as deep, ulcerating lesions with well-defined violaceous or blue borders; however, this entity has 10 distinct variants that have slightly different gross and microscopic features.1,10 Neutrophilic folliculitis, which was diagnosed in our patient, is one form of pustular pyoderma gangrenosum and most typically presents as large, discreet, painful pustules.1 Treatment options for pyoderma gangrenosum include azathioprine, cyclosporine, cyclophosphamide, intralesional steroid injections and hyperbaric oxygen.8
Another rare extra-intestinal manifestation seen in our patient was pyostomatitis vegetans. This gingival disorder, which is highly specific for IBD, is characterized by pustules and erosions within the oral mucosa.2 Pyostomatitis vegetans has also been reported in patients with relatively quiescent bowel symptoms, similar to our case.11 In contrast, strawberry gingivitis, which is associated with GPA, is due to gum hypertrophy and petechial hemorrhage.2
On visual inspection, these distinct conditions can appear similar; therefore, biopsy and clinical context are required to differentiate between pyostomatitis vegetans and strawberry gingivitis.
In terms of management for pyostomatitis vegetans occurring without associated gastrointestinal flare, topical corticosteroids or topical tacrolimus can be used.12,13 However, this symptom tends to be recalcitrant to treatment, and most cases will require systemic steroids.12 Other pharmaceutical options include dapsone, cyclosporin, azathioprine and sulfasalazine.12
Pulmonary involvement in IBD was first described by Kraft et al. in 1976.14 More recently, Storch et al. summarized more than 400 cases of patients with IBD and respiratory disease.15 They categorized these pulmonary manifestations within groups, such as airway disease, interstitial disease, overlapping syndromes and pulmonary vasculitis, similar to GPA.
Most commonly, patients present with non-specific symptoms, such as cough and dyspnea.10 A large portion of patients also have subclinical lung disease, based on studies using screening with pulmonary function tests.16 However, tracheal involvement in IBD, as seen in our patient, remains rare, with only a few other cases reported in the literature.17-19 Tracheal manifestations of IBD can range from asymptomatic tracheal thickening incidentally found on imaging to life-threatening disease. In a majority of circumstances, patients are often unwell with symptoms of hemoptysis, dyspnea, dysphonia and respiratory distress.10 GPA is, interestingly, often part of the differential diagnosis given the dramatic and similar clinical presentation. Inflammation within the trachea can be severe and lead to complications, such as tracheal stenosis, obstruction, rupture and death.10 Many patients with IBD and tracheal involvement require high doses of steroids, bronchodilators and admission to an intensive care unit for airway protection.10
Conclusion
In conclusion, IBD has many extra-intestinal manifestations that can mimic GPA. In our case, specific, rare, cutaneous, gum and tracheal manifestations initially suggested a possible diagnosis of GPA-IBD overlap. However, our patient’s symptoms could ultimately be explained by his underlying IBD. Other factors that pointed away from a diagnosis of GPA-IBD overlap included inconsistent ANCA serology, evidence of active bowel disease on endoscopy and pathology, and multiple biopsies failing to demonstrate histological evidence of vasculitis. Therefore, in conjunction with the various specialists involved in his care, our patient had been been determined to have IBD with rare extra-intestinal manifestations.
In managing IBD, it is vital to work closely with other specialists to determine the true etiology of symptoms. Gastroenterologists, in particular, play an important role, ensuring bowel disease activity is suppressed prior to considering overlap syndromes.
The distinction between GPA-IBD overlap and extra-intestinal manifestations of IBD is important because treatment and prognosis can differ.
Julia Jing-ou Tan, MD, is on the faculty of medicine at the University of British Columbia, Vancouver, Canada.
Mohammad Bardi, MD, is on the faculty of medicine in the Division of Rheumatology, Department of Medicine, University of British Columbia.
Natasha Dehghan, MD, is on the faculty of medicine in the Division of Rheumatology, Department of Medicine, University of British Columbia.
Disclosures: Written patient consent was obtained. However, ethics approval was not sought because the University of British Columbia Clinical Research Ethics Board “does not consider a case report to meet the definition of research; this is considered to be a medical/educational activity.” No conflicts of interest or funding sources were disclosed.
References
- Ruhl AP, Ganz JE, Bickston SJ. Neutrophilic folliculitis and the spectrum of pyoderma gangrenosum in inflammatory bowel disease. Dig Dis Sci. 2007 Jan;52(1):18–24.
- Heera R, Choudhary K, Beena VT, Simon R. Strawberry gingivitis: A diagnostic feature of gingival Wegener’s granulomatosis! Dent Res J (Isfahan). 2012 Dec;9(Suppl 1):S123–S126.
- Sy A, Khalidi N, Dehghan N, et al. Vasculitis in patients with inflammatory bowel diseases: A study of 32 patients and systematic review of the literature. Semin Arthritis Rheum. 2016 Feb;45(4):475–482.
- Humbert S, Guilpain P, Puéchal X, et al. Inflammatory bowel diseases in anti-neutrophil cytoplasmic antibody-associated vasculitides: 11 retrospective cases from the French Vasculitis Study Group. Rheumatology (Oxford). 2015 Nov;54(11):1970–1975.
- Vaszar LT, Orzechowski NM, Specks U, et al. Coexistent pulmonary granulomatosis with polyangiitis (Wegener granulomatosis) and Crohn disease. Am J Surg Pathol. 2014 Mar;38(3):354–359.
- Shaik I, Matta J, Khan R, et al. ANCA negative limited GPA (Wegener’s) of the lung in the setting of severe ulcerative colitis. Chest. 2014 Oct;146(4 Suppl 2):465A.
- Kedziora JA, Wolff M, Chang J. Limited form of Wegener’s granulomatosis in ulcerative colitis. Am J Roentgenol Radium Ther Nucl Med. 1975 Sep;125(1):127–133.
- Shankar S, Sterling JC, Rytina E. Pustular pyoderma gangrenosum. Clin Exp Dermatol. 2003 Nov;28(6):600–603.
- Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011 Jan;106(1):110–119.
- Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ. 2006 Jul 22;333(7560):181–184.
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999 Mar;87(3):327–330.
- Hegarty AM, Barrett AW, Scully C. Pyostomatitis vegetans. Clin Exp Dermatol. 2004 Jan;29(1):1–7.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005 Apr;52(4):722–723.
- Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976 Apr;136(4):454–459.
- Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003 Mar;9(2):104–115.
- Herrlinger KR, Noftz MK, Dalhoff K, et al. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002 Feb;97(2):377–381.
- Ji X-Q, Wang LX, Lu DG. Pulmonary manifestations of inflammatory bowel disease. World J Gastroenterol. 2014 Oct 7;20(37):13501–13511.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol. 2011 Apr;7(4):235–241.
- Dy RV, Xu B, Dhillon SS. Inflammatory tracheobronchitis related to ulcerative colitis. J Bronchol Interv Pulmonol. 2018 Apr;25(2):137–140.