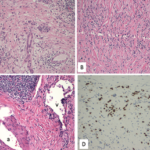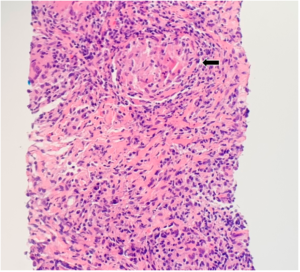
FIGURE 2: CT-guided lung biopsy specimen demonstrating non-necrotizing granuloma (arrow; magnification 100x).
Reviewing the literature, we found a few case reports linking etanercept to new-onset sarcoidosis. Subsequently, we discontinued etanercept for our patient. Treatment with prednisone was initiated, at a dose of 1 mg/kg, which was gradually tapered over the course of eight weeks.
Six weeks after receiving the corticosteroid treatment, the patient presented to follow-up, feeling much improved. A chest X-ray showed near-complete resolution of the left upper lung density, with residual opacity remaining. Six months after this admission, the patient reported complete resolution of her clinical symptoms. CT of the chest revealed complete resolution of bilateral pulmonary infiltrates with minimal left upper lobe scar (see Figure 3).
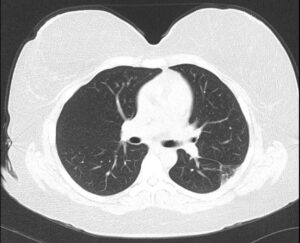
FIGURE 3: Six-month follow-up CT of the chest without contrast: Resolution of bilateral pulmonary infiltrates with minimal left upper lobe scar.
Discussion
Sarcoidosis is a chronic systemic granulomatous disease of unknown etiology.1 The disorder most commonly affects the lungs and lymph nodes, but can involve any organ system.2 In more than 90% of affected patients, intrathoracic involvement can be seen, typically presenting as lymphadenopathy and interstitial lung disease.6 Nonspecific constitutional manifestations may occur, such as fever, fatigue, malaise and weight loss.1 Respiratory symptoms include cough, shortness of breath and pleurisy.7
The diagnosis is established when the following three criteria are present: compatible clinical and radiographic findings, histological evidence of noncaseating granulomas and exclusion of other diseases.8
Sarcoidosis is thought to result from exposure to an unidentified antigen in a genetically predisposed host that leads to an inflammatory response composed of activated macrophages and T lymphocytes.1 These cells produce a pattern of inflammatory cytokines, such as interleukin 2, interferon-γ and TNF-α.1 The production of TNF-α from alveolar macrophages participates in the induction and maintenance of granuloma formation.3 Due to the essential role of TNF-α in the disease’s pathogenesis, TNF-α antagonists are considered part of the treatment of sarcoidosis.3
Paradoxically, several documented cases of sarcoidosis are associated with the use of TNF-α inhibitors, including neurosarcoidosis, cutaneous sarcoidosis and pulmonary sarcoidosis.9-12 The pathophysiology of TNF-α inhibitor-induced sarcoid-like reactions is not entirely understood, but several potential mechanisms have been proposed. It has been suggested that TNF-α suppression can lead to cytokine imbalance, thereby leading to granulomatous inflammation.13 Also, peripheral TNF-α antagonism may activate autoreactive T cells, inducing the formation of granulomas.14