Methotrexate (MTX) remains the predominant medication used by rheumatologists to treat rheumatoid arthritis (RA). Doses of 7.5–25 mg per week with daily folic acid are generally prescribed. Despite its common use, MTX must be prescribed cautiously given the potential adverse effects when taken incorrectly or without folic acid supplementation.
Cases of MTX-induced cutaneous ulceration have been documented mainly in patients with psoriasis. Below, I report on the case of a patient with seropositive RA who developed cutaneous lesions on her hands and lower legs after incorrect use of MTX (see Image 1, above).
The Case
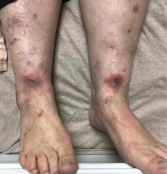
Image 1: Both of the patient’s lower legs and feet featured lesions.
A 65-year-old female with a past medical history of gastroesophageal reflux disease, hypertension and RA (positive tests for anti-cyclic citrullinated peptide [anti-CCP] antibodies and rheumatoid factor) presented at the hospital with a two- to three-week history of sudden-onset abdominal pain, diarrhea, skin rashes and oral ulcers.
She reported that her dose of MTX had been increased from four tablets a week (10 mg) to seven tablets a week (17.5 mg) two months earlier. She had been on MTX for roughly three years and reported compliance with daily folic acid intake. The patient said that when she was on four tablets of MTX weekly, she would take one tablet every other day. Then, when her dose was increased to seven tablets, she took one tablet daily instead of one weekly dose. She had never taken all the tablets at once. Of note, her prescription was actually for six tablets, not seven, to be taken in a single weekly dose.
Her other medications included aspirin daily (81 mg), prednisone daily (5 mg), omeprazole, amlodipine, atorvastatin and valsartan. Of note, the patient did not have a history of psoriasis.
Examination of her skin revealed erythematous indurated plaques with central ulceration and crusting on her lower legs, and similar, smaller lesions on the dorsum of both hands. The patient’s mouth was sore and inflamed, particularly her lower lip and underneath her tongue (stomatitis).
Upon admission, her labs were significant for anemia, with hemoglobin at 9.6 g/dL, and thrombocytopenia, with a platelet count of 76 K/uL. Due to suspicion for MTX toxicity, the primary team gave the patient a 5 mg dose of folinic acid (Leucovorin) orally and started her on folic acid at 1 mg intravenous (IV) daily.
On her second day of admission, the hematology/oncology service evaluated the patient. The physicians believed all of her symptoms were likely related to MTX toxicity and started the patient on 10 mg folinic acid every eight hours for two days. She was also continued on 1 mg of oral folic acid daily.
A dermatologist evaluated her skin lesions, and the differential diagnoses included neutrophilic dermatosis, infection and vasculitis. The dermatologist was less suspicious for an infectious etiology (such as deep fungal or atypical mycobacteria) given how widespread and multifocal the lesions were and the lack of systemic symptoms, such as fever. A punch biopsy was taken and stained with hematoxylin and eosin, and the patient was pan-cultured. Skin biopsy findings revealed cutaneous ulceration due to MTX toxicity.
Over the next three days, the patient’s diarrhea and abdominal pain improved. No new skin or oral lesions developed. Her platelet count started to improve.
Due to symptomatic improvement, patient was discharged after five days in the hospital. She was advised to discontinue MTX and continue daily folic acid at 1 mg daily. Her symptoms continued to improve, and the skin lesions disappeared within two to three weeks of discharge.