crystal light / shutterstock.com
Calciphylaxis is a poorly understood and life-threatening ischemic vasculopathy characterized by calcification of the small- and medium-size arteries in the skin, subcutaneous tissue and internal organs, which leads to thrombosis, tissue necrosis and painful skin ulcerations that won’t heal. The disease has a 50–80% mortality rate. Although affected patients typically have end-stage renal disease (ESRD) and associated hyperparathyroidism, it can (rarely) occur in non-uremic patients. As of now, only a handful of reported cases of autoimmune disease patients with non-uremic calciphylaxis exist.
Below, we present the case of a 25-year-old female with systemic lupus erythematosus (SLE) and class I lupus nephritis with preserved renal function (GFR>100) who had biopsy-proved calciphylaxis. Despite comprehensive, multidisciplinary efforts and the use of numerous different treatment modalities over a three-month hospitalization period, the disease continued to progress, eventually leading to sepsis and death.
We hope this case raises awareness of this rare, fatal disease in the setting of an underlying autoimmune disease and provides a comprehensive overview of the disease, with an emphasis on the treatment modalities currently available.
In patients with chronic kidney disease, calciphylaxis is widely thought to be secondary to the disruption of calcium homeostasis due to secondary hyperparathyroidism.
The Case Report
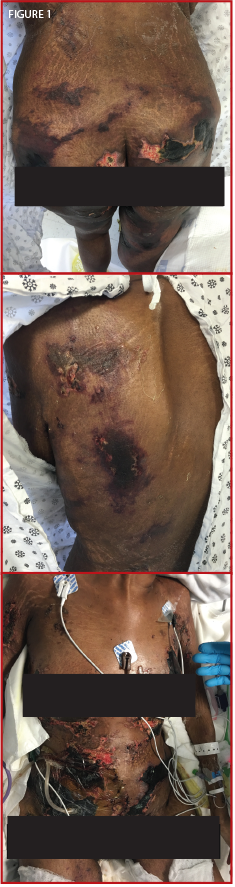
Figure 1. The patient presented confluent, hyperpigmented plaques over her thighs, buttocks, back, breasts and abdomen.
A 25-year-old black female with a two-year SLE history with features of arthritis, discoid skin lesions and class I lupus nephritis presented with a two-week history of hyperpigmented skin lesions on her arms, thighs, chest and back, associated with worsening pain. Her immunosuppressive regimen at the time consisted of mycophenolate mofetil 1,500 mg BID, hydroxychloroquine 400 mg QD, prednisone 5 mg QD and monthly belimumab infusions. Examination revealed confluent hyperpigmented plaques distributed across her upper extremities, thighs, chest and back (see Figure 1).
Laboratory data upon initial review were significant for profound anemia, with a hemoglobin of 6.3 g/dL and hematocrit of 21%. Her erythrocyte sedimentation rate (ESR) was elevated to 118 mm/h with a C-reactive protein (CRP) of 1.5 mg/dL; lactate dehydrogenase (LDH) and haptoglobin remained within normal limits. DsDNA was elevated at 193.9 IU, C4 was low at 5 mg/dL and C3 was normal at 94 mg/dL. However, when compared with her previous lupus serologies, the complements and dsDNA were close to her baseline. Otherwise, serum creatinine (0.8 mg/dL), calcium (10.3 mg/dL), phosphorus (3.7 mg/dL), glomerular filtration rate (GFR), liver function tests and the rest of her basic metabolic panel proved unremarkable (see Table 1).