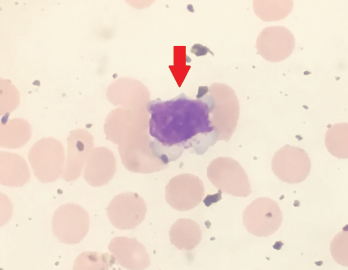
Figure 1. Peripheral blood smear showing a large granular lymphocyte in a 53-year-old Hispanic man.
Large granular lymphocytic (LGL) leukemia is a rare, chronic, lymphoproliferative disorder of cytotoxic T cell or natural killer cell lineage with an annual incidence of 0.72 cases per 1 million people in the U.S.1 The most common subtype of LGL leukemia, T-LGL leukemia, follows an indolent disease course and accounts for approximately 85% of cases. A second subtype, the chronic, lymphoproliferative disorder of natural killer cells (CLPD-NK), accounts for less than 10% of cases. The third subtype, known as aggressive natural killer-LGL leukemia, accounts for less than 5% of cases and carries a poor prognosis because it is refractory to chemotherapy.2,3
The diagnosis is made by first identifying an increased population of circulating large granular lymphocytic cells. An absolute lymphocyte count of >2,000 lymphocytes/mL may be helpful; however, it’s not mandatory because the diagnosis can be made if a clonal large granular lymphocytic population is identified in the clinical setting of autoimmune disease, such as rheumatoid arthritis or cytopenias (e.g., neutropenia, anemia, thrombocytopenia).2,4
Rheumatoid arthritis (RA) is one of the most common autoimmune disorders in developed countries, with prevalence estimates of approximately 0.5–1%.5 This chronic, systemic, inflammatory disorder is characterized by symmetric polyarticular synovial inflammation and joint destruction. RA has long been associated with LGL leukemia. Up to one-third of patients diagnosed with LGL leukemia will have a concomitant diagnosis of RA.2,4
RA is a known risk factor for the development of osteoporosis and vertebral fractures; however, little is known about the risk of osteoporosis and fractures in LGL leukemia. Here, we present a case of a patient with RA complicated by the sudden onset of LGL leukemia, as well as the accelerated development of multiple vertebral fractures.
Case Presentation
A 53-year-old Hispanic man with a 15-year history of high-titer, rheumatoid factor positive, anti-citrullinated protein antibody (ACPA) positive, nonerosive RA was seen in the outpatient rheumatology clinic for an RA flare. He complained of tenderness and swelling of both wrists, the metacarpophalangeal and proximal interphalangeal joints. He also reported a decreased ability to perform activities of daily living.
The patient had a long history of treatment-resistant RA, with treatment failures including hydroxychloroquine, sulfasalazine, adalimumab, etanercept, abatacept and tocilizumab. The decision was made to initiate certolizumab for management of his RA.
Five weeks after the initiation of certolizumab, he developed a new, severe neutropenia, with an absolute neutrophil count of 300 cells/μL. A peripheral smear showed atypical, large lymphocytes (see Figure 1), as well as smudge cells.
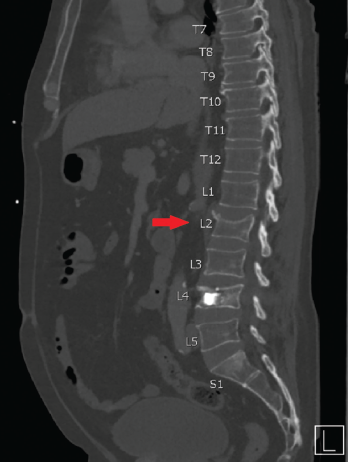
Figure 2. CT scan showing an incidental, non-traumatic L2 vertebral compression fracture.
The patient’s neutropenia persisted despite discontinuation of certolizumab. Peripheral flow cytometry showed an increased population of large T cell granulocytes. A T cell gene rearrangement study was ordered, which confirmed the diagnosis of LGL leukemia. Oral methotrexate in addition to low-dose methylprednisolone (i.e., 4 mg twice a day) was initiated to treat both the LGL leukemia and the RA, with subsequent improvement of neutropenia.
Methotrexate therapy had not previously been trialed due to his history of hepatic steatosis and liver enzyme elevations.
Four months after initiation of therapy, the patient suffered a traumatic L4 vertebral compression fracture after lifting a heavy case of bottled water. This was managed with L4 kyphoplasty.
Two weeks following kyphoplasty, an incidental non-traumatic L2 vertebral compression fracture was found on computed tomography (CT) scan performed for abdominal pain (see Figure 2). Dual-energy X-ray absorptiometry (DXA) imaging showed a T-score of -1.5 and a low fracture risk assessment (FRAX) of 13.8%/1.0%.
His clinical course was complicated by repeated hospitalizations over the next two months for complications of cholecystitis, during which time multiple CT scans obtained for evaluation revealed rapid interval progression of compression fractures.
Lumbar spine plain radiographs obtained two months after the first compression fracture showed a striking multilevel array of compression fractures involving T10 through L5 (see Figure 3).
Repeated urine and blood testing for paraproteins were negative. He was treated with teriparatide for multilevel pathologic vertebral fractures.
Up to one-third of patients diagnosed with LGL leukemia will have a concomitant diagnosis of RA.
Discussion
Historical aspects of LGL leukemia
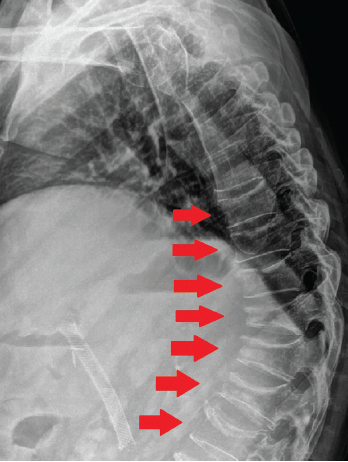
Figure 3. Plain radiograph of the thoracic and lumbar spine two months after discovery of the incidental L2 vertebral compression fracture on CT imaging. Shown here is a striking multilevel array of compression fractures involving T10–L5.
LGL leukemia is a rare, chronic, lymphoproliferative disorder of cytotoxic T cell or natural killer cell lineage. It was initially described in 1975 by Brouet et al., who identified 11 patients with chronic lymphocytic leukemia (CLL) of T cell origin with lymphocytes demonstrating possible clonal expansion.6
In 1977, McKenna et al. noted a small cohort of patients with chronic lymphocytic leukemia having unusual membrane surface receptors reacting to sheep erythrocytes, a marker of T lymphocytes.7
In 1985, Loughran et al. went on to demonstrate the presence of clonal cytogenetic abnormalities suggesting the disease process originated from a clonal proliferation of immature natural killer cells.4
Taken together, these cytogenic and immunologic findings from Brouet, McKenna and Loughran identified LGL leukemia as a separate, clinically and pathologically distinct neoplastic entity consisting of a lymphocytosis with clonal expansion of large granular lymphocytes.4,6,7
Two subsets of LGL leukemia were proposed in 1993: T cell large granular lymphocytic and a more aggressive NK-cell leukemia. The World Health Organization (WHO) recognized this classification scheme in 2001, with an additional provisional change in 2008 after the identification of a third subset of chronic NK-cell lymphocytosis, which was less aggressive than NK-cell leukemia.2,4
Hematologic malignancies & vertebral compression fractures
The prevalence of decreased bone marrow density and vertebral fracture risk in LGL leukemia is largely unknown, possibly due to the rarity of the neoplastic disorder. However, data can be gleaned from other forms of hematologic malignancies.
In 2016, Olszewski et al. conducted a large, retrospective, case-control study using the Surveillance, Epidemiology and End Results registry linked to Medicare claims (SEER-Medicare) to evaluate the vertebral and pelvic fracture risk in patients with untreated chronic lymphocytic leukemia. The group compared fracture data from 16,344 chronic lymphocytic leukemia cases to 114,408 cases matched by age, sex, race and reason for Medicare enrollment (age vs. disability). There was a significantly higher risk of axial fractures among chronic lymphocytic leukemia patients compared with controls, with an overall hazard ratio of 1.37, confidence interval of 1.25–1.50 and P<0.001.8
In 2009, Halton et al. performed a prospective evaluation to identify the prevalence, location, severity and morphology of thoracic and lumbar vertebral fractures in children with newly diagnosed acute lymphocytic leukemia (ALL). The group evaluated 186 newly diagnosed children with acute lymphocytic leukemia within the first 30 days of diagnosis for the presence of vertebral compression fractures and bone mineral density (BMD). Researchers found 29 patients (16%) with an overall total of 75 vertebral fractures (53 thoracic, 22 lumbar). BMD was found to be affected in all of the children with new acute lymphocytic leukemia, regardless of vertebral fracture status, with a mean reduction in Z-score of -1.2. Interestingly, 45% of patients with vertebral fractures were asymptomatic.9
LGL leukemia & vertebral compression fractures
Data from studies of both chronic lymphocytic leukemia and acute lymphocytic leukemia have implicated these hematologic malignancies as a risk factor for reductions in vertebral BMD and vertebral compression fractures, with increasing risk of compression fractures also correlating with loss in vertebral bone mineral density.8,9 However, not much data regarding vertebral compression fractures of LGL leukemia patients exist.
Murine models of LGL leukemia have shown similar decreases in bone mineral density. In 1990, Rosol et al. studied the effects of LGL leukemia on trabecular bone remodeling in F344 rats. These rats were transplanted with high-passage LGL cells, which resulted in the development of LGL leukemia. Lumbar vertebrae exams were performed every three days up to 12 days in the murine models to evaluate for changes in trabecular bone formation. Rats with LGL leukemia developed significant decreases in trabecular bone formation and reduction in trabecular bone associated with infiltration of the intramedullary space with LGL cells.10
One recent case report described a 54-year-old woman with no history of chronic illness who was diagnosed with LGL leukemia and later found to have an incidental finding of an old L1 vertebral body fracture on magnetic resonance imaging obtained due to lumbosacral junction tenderness. This patient was later found to have a rare phenotype of LGL leukemia (CD4/CD8 double-positive) with the development of an associated multiple myeloma. CT imaging of the thorax, abdomen and pelvis showed infiltrative bone marrow disease.11
In our reported case, the patient developed rapidly progressive multilevel vertebral compression fractures within six months of diagnosis of LGL leukemia, despite a low FRAX score. Based on the available data of other infiltrative hematologic malignancies and known cases of LGL leukemia-related compression fractures, we postulate there may be a link between LGL leukemia and an increased risk of vertebral fractures.
LGL leukemia & pathogenic mechanism of vertebral compression fractures
The mechanisms by which these vertebral fractures occur are complex and multifactorial. One possible contributory mechanism is leukemia-associated microenvironmental changes. In chronic lymphocytic leukemia, as leukemia infiltration of the bone marrow progresses, interactions between the microenvironment and leukemia cells weaken the cancellous bone structure via the release of osteoclast-activating factors, there is an increased expression of RANKL on chronic lymphocytic leukemia cells, leading to an increase in RANKL receptor activation, osteoclast differentiation and activity leading to bony structural damage.
Further, there is an upregulation of cytokines interleukin (IL) 6, IL-8 and tumor necrosis factor (TNF) α in patients with chronic lymphocytic leukemia which serves to further stimulate osteoclast differentiation and activation.8,12
Evaluation of cytokines in LGL leukemia patients show similar elevations in inflammatory cytokine profiles.2,13 In addition to its effects on osteoclast differentiation and activation, TNF-α is also a potent stimulator of Dickkopf-1 (DKK-1), a protein that inhibits the WNT pathway, therefore inhibiting osteoblast differentiation and function.14 Together, these changes result in both increased bone resorption and decreased bone formation.
However, LGL leukemia alone does not likely explain the rapid rate of vertebral fractures in our patient. We suspect our patient had such a rapid rate of compression fracture development due to the additive effects of RA and low-dose steroid use on vertebral fracture risk.
A meta-analysis of two cohort studies, two nested case-control studies and three case-control studies encompassing 631,210 participants with RA showed a significant increase in vertebral fractures with a relative risk of 2.34 (95% CI 2.05–2.63). A subgroup analysis of the study by sex showed a similar relationship, despite male or female gender.3
In addition, even at doses of 2.5 mg/day to 7.5 mg/day of prednisolone or equivalent, corticosteroid use has been associated with an increased relative rate of vertebral fractures of 1.62 when compared with age-matched controls (95% CI, 1.29–1.84).15
We suspect the additive effects of LGL leukemia, RA and low-dose steroid use on the vertebrae microenvironment resulted in the unusually rapid progression of vertebral fractures in our patient, despite a T-score at the time of the first fracture showing only mild osteopenia.
Conclusion
We present a case of rapidly progressive vertebral compression fractures in a patient with a history of RA and recently diagnosed LGL leukemia. We suspect that, similar to other infiltrative malignancies, such as acute lymphocytic leukemia and chronic lymphocytic leukemia, LGL leukemia may increase the risk of vertebral compression fractures. Additional studies may help further delineate this risk.
 Maj. Nam D. Nguyen, DO (USAF, MC), is a rheumatology fellow at the San Antonio Uniformed Services Health Education Consortium, Fort Sam Houston, Texas.
Maj. Nam D. Nguyen, DO (USAF, MC), is a rheumatology fellow at the San Antonio Uniformed Services Health Education Consortium, Fort Sam Houston, Texas.
 Lt. Col. Erica Hill, DO (USAF, MC), is a rheumatologist in the Department of Rheumatology, Brooke Army Medical Center, Fort Sam Houston, Texas.
Lt. Col. Erica Hill, DO (USAF, MC), is a rheumatologist in the Department of Rheumatology, Brooke Army Medical Center, Fort Sam Houston, Texas.
 Col. Jay Higgs, MD (ret., USAF, MC), is a rheumatologist in the Department of Rheumatology, Brooke Army Medical Center, Fort Sam Houston, Texas.
Col. Jay Higgs, MD (ret., USAF, MC), is a rheumatologist in the Department of Rheumatology, Brooke Army Medical Center, Fort Sam Houston, Texas.
Key Takeaways
- Large granular lymphocytic (LGL) leukemia is a rare chronic lymphoproliferative disorder and up to one-third of patients have a concomitant diagnosis of rheumatoid arthritis;
- The risk of LGL leukemia in the development of osteoporosis and vertebral fractures is largely unknown, likely due to the rarity of the disorder; and
- An increase in RANKL receptor activation and upregulation of cytokines IL-6, IL-8 and TNF-α may contribute to the pathogenesis of LGL leukemia-associated vertebral compression fractures.
References
- Gazitt T, Loughran TP Jr. Chronic neutropenia in LGL leukemia and rheumatoid arthritis. Hematology Am Soc Hematol Educ Program. 2017 Dec 8;(1):181–186.
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: From pathogenesis to treatment. Blood. 2017 Mar 2;129(9):1082–1094.
- Oshimi K. Clinical features, pathogenesis, and treatment of large granular lymphocyte leukemias. Intern Med. 2017;56(14):1759–1769.
- Loughran T, Starkebaum G. Large granular lymphocyte leukemia: Report of 38 cases and a review of the literature. Medicine (Baltimore). 1986 Sep;66(5):397–405.
- Shah MV, Hook CC, Call TG, et al. A population-based study of large granular lymphocyte leukemia. Blood Cancer J. 2016 Aug 5;6(8):e455.
- Brouet JC, Sasportes M, Flandrin G, et al. Chronic lymphocytic leukaemia of T-cell origin. Immunological and clinical evaluation in eleven patients. Lancet. 1975 Nov 8;2(7941):890–893.
- McKenna RW, Parkin J, Kersey J, et al. Chronic lymphoproliferative disorder with unusual clinical, morphologic, ultrastructural and membrane surface marker characteristics. Am J Med. 1977 Apr;62(4):588–596.
- Olszewski AJ, Gutman R, Eaton C. Increased risk of axial fractures in patients with untreated chronic lymphocytic leukemia: A population-based analysis. Haematologica. 2016 Dec;101(12):488–491.
- Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: Results of the Canadian steroid-associated osteoporosis in the pediatric population (STOPP) research program. J Bone Miner Res. 2009 Jul;24(7):1326–1334.
- Rosol TJ, Stromberg PC. Effects of large granular lymphocyte leukemia on bone in F344 rats. Vet Pathol. 1990 Nov;
27(6):391–396.
- Soliman D, Sallam S, Akiki S, et al. T-cell large granular lymphocytic leukemia with extremely rare immunophenotype (CD4/CD8 double-positive) followed by multiple myeloma diagnosis. Case Rep Hemotol. 2020 Aug 14;2020:8839144.
- Marini C, Bruno S, Fiz F, et al. Functional activation of osteoclast commitment in chronic lymphocytic leukaemia: A possible role for Rank/RANKL pathway. Sci Rep. 2017 Oct 26;7(1):14159.
- Shvidel L, Duksin C, Tzimanis A, et al. Cytokine release by activated T-cells in large granular lymphocytic leukemia associated with autoimmune disorders. Hematol J. 2002;3(1):32–37.
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007 Feb;13(2):156–163.
- Van Staa TP, Leufkens HGM, Abenhaim L, et al. Oral corticosteroids and fracture risk: Relationship to daily and cumulative doses. Rheumatology (Oxford). 2000 Dec;39(12):1383–1389.
Authors’ Note
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense or the U.S. government.
