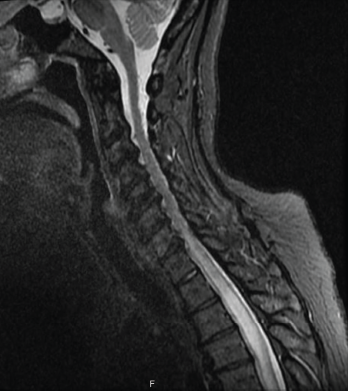
Figure 1: MRI of the spine
Sjögren’s syndrome is a chronic multi-system autoimmune disease characterized by inflammation and subsequent destruction of exocrine glands. Sjögren’s syndrome can present with glandular or extra-glandular manifestations. Neuromyelitis optica spectrum disorder (NMOSD) is a rare central nervous system (CNS) autoimmune disease that can present as the initial manifestation in less than 5% of patients with Sjögren’s.
It is important to recognize relationships between autoimmune diseases, especially Sjögren’s syndrome and NMOSD. This rare association requires a high degree of suspicion in a patient with Sjögren’s syndrome with a suggestive clinical presentation and imaging findings.
Here, we present an interesting case of NMOSD in a Sjögren’s syndrome patient.
Case Presentation
A 51-year-old African American woman with a history of lip-biopsy proven primary Sjögren’s syndrome (with sicca, positive anti-nuclear antibody, positive Sjögren’s A/Ro and positive Sjögren’s B/La, lymphocytic interstitial pneumonitis and non-inflammatory myopathy) presented to the emergency department with two days of worsening lower extremity weakness, acute bladder and bowel incontinence, and blurry vision.
On physical examination, she had bilateral lower extremity flaccid paralysis, absent bilateral patellar and Achilles tendon reflexes, and loss of sensation for pain, temperature, light touch, vibration and joint position sense below the T4 level. All other system reviews were unremarkable.
Magnetic resonance imaging (MRI) of the spine indicated intra- and extramedullary spinal cord enhancement from T3–T11, and edema from C5–T11 (see Figure 1). An MRI of the brain revealed a 2 cm T1 hypointense, T2/FLAIR hyperintense periventricular lesion in the left frontoparietal centrum semiovale with mild perivascular enhancement. An orbital MRI suggested an increased signal within the right optic nerve without focal enhancement, consistent with optic neuritis (see Figure 2).
Her cerebrospinal fluid (CSF) analysis showed a white blood cell count of 1,300/uL (normal: 0–5/uL) with 88% neutrophils and elevated CSF protein of >300 mg/dL (normal: 15–45 mg/dL). Other CSF studies—including acid-fast bacilli, bacterial and fungal culture, venereal disease research lab, herpes simplex virus, cryptococcus, West Nile virus, Epstein-Barr virus, toxoplasma, blastomyces, histoplasma, cytomegalovirus and varicella tests—were negative. CSF oligoclonal bands, serum and CSF lymphoma panels, and a positron emission tomography scan were also normal. Serum aquaporin-4 (AQP4) immunoglobulin G antibodies (IgG) were elevated at 362.1 U/mL (normal: 0–3 U/mL).
Based on the 2015 revised criteria by the International Panel for NMO Diagnosis (IPND, see Table 1), a diagnosis of NMOSD was made.
The patient was initially treated with intravenous dexamethasone and tapered to 40 mg of oral prednisone daily. She received IV antibiotics due to an initial concern for infection, which was discontinued when her CSF culture proved negative. Additionally, she underwent five cycles of plasmapheresis. Her weakness did not improve during hospitalization, so she was transitioned to acute rehab to follow up in the rheumatology clinic for initiation of subcutaneous tocilizumab.