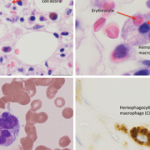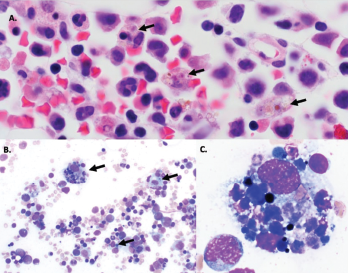
Figure 1A–C: Arrows denote hemophagocytic histiocytes.
Figure A: Hematoxylin and eosin stained bone marrow biopsy (1,000X).
Figure B: May-Gurenwald-Giemsa bone marrow aspirate (100X).
Figure C: May-Gurenwald-Giemsa bone marrow aspirate (1,000X).
COG4 mutations have previously been reported in two patients with autosomal recessive COG4-CDG (CDG-IIj), who were described as having a similar set of clinical symptoms of hypotonia, seizures, coagulopathy and liver dysfunction, as well as recurrent infections.
Over the next three months, the patient experienced two more episodes of fever, liver dysfunction, coagulopathy and shock physiology. The second episode was preceded by a similar set of symptoms of fever, vomiting and diarrhea, and was successfully treated with FFP.
The third episode occurred four days after he received hepatitis A and measles mumps rubella (MMR) vaccinations at 12 months. Our patient again required intensive care and had prolonged hypotension requiring inotropic support. His hypotension resolved after receiving daily FFP, and his hypoxia resolved after a single treatment with protein C concentrate.
Discussion
Congenital disorders of glycosylation are inborn metabolism errors characterized by defects in glycosylation (i.e., the assembly, processing and addition of sugars to proteins and lipids). The two main types of glycosylation are N-linked and O-linked glycosylation.
N-linked glycosylation is the most common covalent protein modification, in which sugars are linked to the nitrogen groups on asparagine residues of proteins in a consensus amino acid sequence (Asn-X-Ser/Thr) with a common pentasaccharide core.1 This modification begins on the cell surface of the endoplasmic reticulum and is completed in the Golgi apparatus.
O-linked glycosylation occurs in the lumen of the endoplasmic reticulum and occurs with the addition of N-acetylgalactosamine (GalNAc) to the hydroxyl group on serine or threonine without the conserved pentasaccharide core.2
Because glycosylation plays an important role in every organ, CDG affects multiple organ systems, including the nervous (causing developmental delays, seizures, ataxia and hypotonia), endocrine, renal and immune systems, as well as the liver (causing transaminitis and coagulopathy).
Looking specifically at the immune system, proper glycosylation is critical for normal immune function. At least 23 CDG patients with immunological involvement, such as an increased propensity to infections and inflammatory and autoimmune complications, have been documented; all of these may demonstrate significant clinical variability associated with the same CDG mutation.3
Manifestations of immune deficiency include recurrent sinopulmonary and gastrointestinal infections, skin abscesses and episodes of bacterial sepsis and invasive fungal infections. Inflammatory and autoimmune manifestations may include an Omenn syndrome-type skin rash, inflammatory bowel disease, celiac disease, autoimmune hypothyroidism, Behçet’s-like disease and familial Mediterranean fever. These manifestations are not mutually exclusive, and patients with immunodeficiency syndromes may also present with autoimmune or inflammatory complications.
With regard to HLH in patients with CDG, case of patients with PMM2-CDG and COG6-CDG presenting with HLH have been reported.4,5 Looking more broadly at patients with inborn metabolism errors, cases of patients with cobalamin C disease, long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD) and lysinuric protein intolerance who presented with HLH have also been reported.6-8
Whether these patients presented with HLH as a direct result of their underlying metabolic disease is not certain. However, it has been hypothesized the increased buildup of toxic metabolites related to the underlying metabolic defect may decrease NK cell function and increase cell expansion and activation of macrophages.9