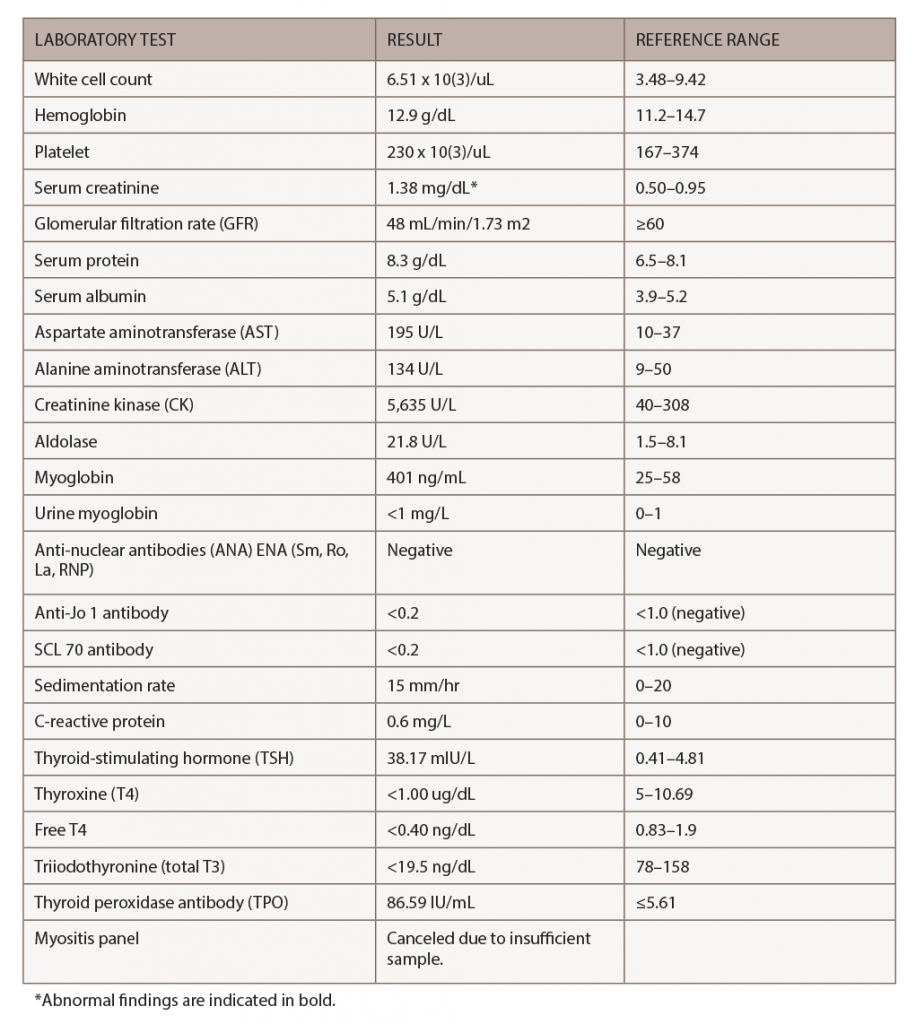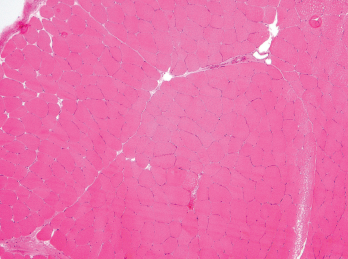
Figure 3. A biopsy of the patient’s left quadriceps muscle. Note the scattered atrophic fibers, mainly type 2. There is no myonecrosis, regeneration, inflammation or significant immunohistochemical upregulation of MHC class I and II.
Although the magnitude of CK elevation observed in this case is unusual for hypothyroid myopathy, it was probably the etiology given the patient’s manual strength testing and that her TSH and CK levels normalized with thyroid replacement. Inflammatory myositis was less likely given her improvement without additional steroids or immunosuppression.
After two months of weight-based levothyroxine, her TSH normalized to 4.3 mIU/L (RR: 0.41–4.81 mIU/L), T4 to 7.5 ug/dL (RR: 5–10.69 ug/dL), free T4 to 2.3 ng/dL (RR: 1.1–2.4 ng/dL), free T3 to 103.7 ng/dL (RR: 78–158 ng/dL) and CK to 82 U/L (RR: 40–308 U/L).
Discussion
IRAEs typically develop within weeks to months after immune checkpoint inhibitor treatment initiation and can affect multiple organ systems.1,2 CTLA-4 acts in lymphoid organs by competing with CD28 binding to CD80/86, the co-stimulatory signal necessary for T cell activation. PD-1 inhibits effector T cells at later stages of the immune response in peripheral tissues.5,6 Patients treated with anti-CTLA-4 and anti-PD-1/PD-1L therapy have distinct IRAEs, with anti-CTLA-4-related IRAE being generally more severe.
The exact mechanisms underlying IRAEs remain elusive. Potential underlying mechanisms resulting in IRAEs include increased T cell activity against antigens that are shared by tumor and healthy tissues, increased levels of preexisting autoantibodies, increased levels of inflammatory cytokines and enhanced complement-mediated inflammation due to direct binding of an anti-CTLA-4 antibody to CTLA-4 expressed on normal tissues, such as the pituitary.1
Endocrinopathies related to immune checkpoint blockade can affect the pituitary gland (e.g., hypophysitis, corticotrophin decrease, secondary adrenal insufficiency), thyroid (e.g., hyperthyroidism, hypothyroidism, thyroiditis), adrenal glands (e.g., primary adrenal insufficiency) and pancreas (e.g., diabetes mellitus).7 Hypophysitis is more commonly reported with anti-CTLA-4 therapy, probably due to anti-CTLA-4 binding to CTLA-4, which is normally expressed in pituitary cells.8
Thyroid dysfunction has been reported in up to 10% of patients treated with PD-1/PD-L1 blockade. In a cohort of patients with non-small-cell lung cancer treated with pembrolizumab (a PD-1 inhibitor), investigators found hypothyroidism was often preceded by a transient and asymptomatic period of hyperthyroidism. Anti-thyroid antibodies were highly and temporally associated with nearly all cases of thyroid dysfunction, suggesting PD-1 inhibitors may unmask latent autoimmunity.9 Our patient had preexisting thyroid antibodies and experienced transient hyperthyroidism prior to developing hypothyroidism and myositis, as evidenced by myalgias, muscle weakness and elevated CK levels.
Hypothyroid myopathy can mimic inflammatory myopathies and should be considered in patients treated with immune checkpoint blockade (ICB) because IRAE endocrinopathies are more common than inflammatory myositis. Patients with frank hypothyroidism have a wide spectrum of muscular symptoms, including stiffness, myalgias, cramps and easy fatigability.10 Elevated serum CK is found in 37–60% patients with hypothyroidism but does not correlate with the severity of myopathic symptoms.
Elevated CK levels (greater than 10 times above the upper limit of normal) may suggest rhabdomyolysis, which is a rare complication of hypothyroidism. Cases of proximal myopathy with a presentation similar to polymyositis involving the shoulders and hips and with an associated CK elevation have been reported.11 Muscle biopsy in hypothyroid myopathy can be normal and can have nonspecific changes, such as type II fiber atrophy. The treatment of choice in hypothyroid myopathy is thyroid hormone replacement.10
Although myalgias and muscle weakness have been reported in clinical trials of participants on immunotherapy, the etiology of these symptoms remains unclear. Immunotherapy-related inflammatory myopathies have been reported.3 In a case series of 654 oncological patients receiving PD-1 inhibitors, five developed myopathy (0.76%), all occurring in patients treated with pembrolizumab.12 Patients developed weakness after a median of two cycles of pembrolizumab treatment and shared common clinical features of oculobulbar and neck flexor weakness. One patient had concomitant acetylcholine receptor (AChR) antibody positive myasthenia gravis. Pathologic findings were varied, and the two patients with necrotizing myopathy had fatal outcomes despite treatment with high-dose steroids and plasma exchange. Interestingly, CK levels did not correlate with disease severity, because the patient with the highest
CK level had a relatively benign
clinical course.12
Physicians in many specialties (including rheumatology) are becoming more aware of IRAEs and creating guidelines for their evaluation and management. Current recommendations for patients with suspected myositis include manual strength testing, dynamometry, blood work (including serologies for CK, aldolase, ESR, CRP, ANA and a myositis panel (e.g., Jo-1, PL-7, PL-12, EJ, OJ, Mi-2, SRP), electromyography and MRI of the affected muscle.3
Because endocrinopathies are relatively common IRAEs and patients with hypothyroidism often have abnormal CK levels, it may prove useful to check thyroid function tests in evaluating patients on immunotherapy who present with myositis.