Immune checkpoint inhibitors (ICIs) targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or programmed cell death protein 1 (PD-1) axes have revolutionized therapy and improved survival in advanced cancers. However, these immune system modulators also lead to immune-related adverse events (IRAEs).1,2
In clinical trials, IRAEs mainly involved the gastrointestinal tract, skin, endocrine glands, liver and lung, but nearly all organs can be affected. Arthralgias, myalgias and arthritis are the most commonly reported rheumatic IRAEs, but there are case reports of polymyalgia rheumatica (PMR), vasculitis, polymyositis/dermatomyositis, lupus nephritis and scleroderma-like reactions.3,4
In this article, we discuss a case of thyroid myopathy in a patient with diffuse pigmented villonodular synovitis (PVNS) treated with durvalumab (a programmed cell death 1 ligand [PD-1L] inhibitor) and an experimental colony-stimulating factor 1 receptor (CSF-1R) inhibitor, PLX3397. After therapy with the PD-1L inhibitor, the patient developed proximal muscle weakness and elevated muscle enzymes; an elevated thyroid-stimulating hormone (TSH) with undetectable thyroid hormones supported a diagnosis of hypothyroid myopathy. Weakness and muscle enzymes improved after thyroid replacement therapy.
This case highlights the importance of considering endocrinopathies in patients on immunotherapies with myopathy because thyroid dysfunction is one of the most common IRAEs.
Case Presentation
A 54-year-old Hispanic woman with a history of hypothyroidism and recurrent, refractory, diffuse PVNS of her left knee presented to our rheumatology department for progressive proximal muscle pain, weakness and an elevated creatinine kinase (CK) level of 5,635 U/L (reference range [RR]: 40–308 U/L).
She had been diagnosed with diffuse PVNS five years prior to presentation after resection of a left knee mass that demonstrated characteristic oval to epithelioid mononuclear and multinucleated histiocytic-like cells with scattered foamy histiocytes and abundant hemosiderin pigment. She was initially treated with a combination of sirolimus and PLX3397, but the disease recurred after three years, and she had another resection before switching to a second experimental CSF-1R inhibitor, LY3022855, and durvalumab.
Thyroid dysfunction has been reported in up to 10% of patients treated with PD-1/PD-L1 blockade.
Her second treatment regimen was discontinued after three months due to persistent psoriasiform eruption with inverse features thought to be IRAE secondary to durvalumab. Her rash responded well to one dose of infliximab infusion and prednisone. Her CK was mildly elevated to 631 U/L at that time.
Seven months after discontinuing durvalumab and LY3022855, she was referred to a rheumatologist for worsening muscle pain, proximal weakness and rising muscle enzymes. She had difficulty standing from a seated position and raising her arms above her head, accompanied by shoulder pain. She also complained of decreased grip strength, causing her to frequently drop objects. Her left knee pain got worse when walking upstairs. She had dyspnea on exertion, a brown rash on her extremities and generalized fatigue. She exhibited no dysphagia or dysphonia, had no trouble holding her head up, did not have chest pain, oral or nasal ulcers, Raynaud’s phenomenon or weight loss.
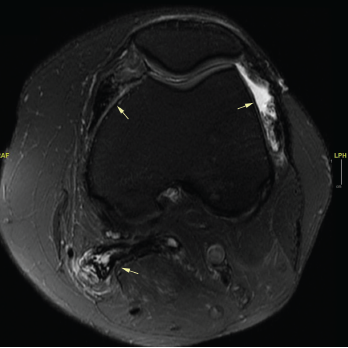
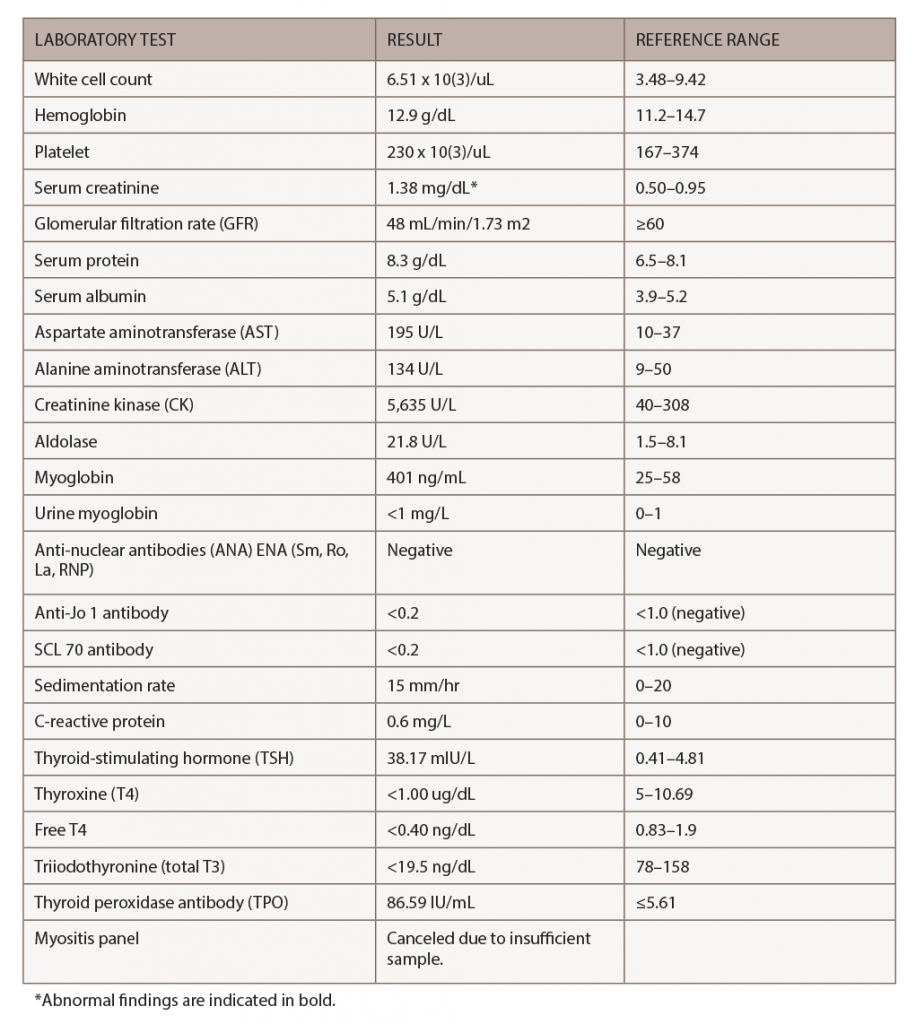
Figure 1. An MRI of the patient’s left knee with and without IV contrast. The knee featured diffuse pigmented villonodular synovitis, including within a lobulated Baker’s cyst, without substantial change in disease burden dating back to 2017.
Her tumor was stable off therapy as demonstrated by magnetic resonance imaging (MRI) of her left knee, which identified diffuse pigmented villonodular synovitis and a lobulated Baker’s cyst without substantial change in disease burden compared to one year ago (see Figure 1).
On physical exam, the patient had normal vital signs and was not in acute distress. She had normal heart and lung sounds, and a soft abdomen that was not tender on palpation. She had a post-inflammatory, hyperpigmented rash on her extremities, but no active lesions. Both shoulders and biceps were diffusely tender to palpation, and her left knee had a moderate joint effusion and tenderness to palpation with an inability to fully extend.
Strength testing revealed 4-/5 muscle strength in neck flexion and extension, 4/5 muscle strength in the proximal upper extremities, 4-/5 muscle strength in bilateral hand grip, 4-/5 muscle strength in bilateral hip flexion, 4/5 muscle strength in knee extension, 4/5 muscle strength in ankle dorsiflexion and 4/5 muscle strength in bilateral toe flexion and extension.
Her laboratory tests during the clinic visit appear in Table 1.
She had no history of neck irradiation or thyroid surgery. One year prior to presentation, she had normal thyroid function tests, but had elevated thyroid peroxidase (TPO) antibodies at 585 mIU/L (RR: ≤5.61 IU/mL) and anti-thyroglobulin antibodies at 77.96 IU/mL (RR: ≤4.11 IU/mL); and normal thyroid-stimulating immunoglobulin at 99% (RR: ≤122%). She developed transient thyroiditis, with palpitations, tremors and low TSH two months after starting durvalumab, and developed hypothyroidism one month after stopping durvalumab. The patient was compliant with levothyroxine (Synthroid) until stopping the medication five months after stopping durvalumab because she misunderstood the instructions.
An MRI of both thighs, with and without contrast, revealed mild diffuse muscle edema in the adductor magnus and semimembranosus muscles bilaterally, worse on the left side (see Figure 2). A muscle biopsy of her left quadriceps on pathological review identified scattered atrophic fibers largely, but not completely, restricted to type 2; there was no evidence of myonecrosis, regeneration or inflammation, and no evidence of significant immunohistochemical upregulation of major histocompatibility complex (MHC) class I and II (see Figure 3).
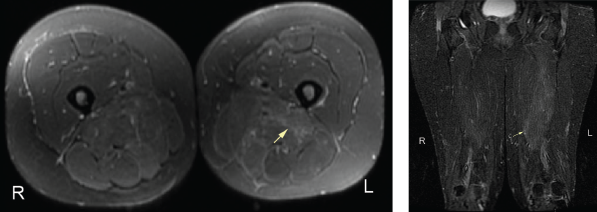
Figure 2. An MRI of the patient’s left and right femur without IV contrast. Note the mild diffuse muscle edema in the adductor magnus and semimembranosus muscles bilaterally, the left greater than right.
The myositis panel was canceled due to an inadequate blood sample.
Differential diagnoses in this case included paraneoplastic myositis secondary to underlying malignancy, inflammatory polymyositis or dermatomyositis as an IRAE from the PD-1L inhibitor, elevated CK as a side effect of the CSF-1R inhibitor, polymyositis associated with infliximab treatment, and hypothyroid-related myopathy as an IRAE from the PD-1L inhibitor. Inflammatory myositis was unlikely given the unremarkable muscle biopsy and normal inflammatory markers.
Figure 3. A biopsy of the patient’s left quadriceps muscle. Note the scattered atrophic fibers, mainly type 2. There is no myonecrosis, regeneration, inflammation or significant immunohistochemical upregulation of MHC class I and II.
Although the magnitude of CK elevation observed in this case is unusual for hypothyroid myopathy, it was probably the etiology given the patient’s manual strength testing and that her TSH and CK levels normalized with thyroid replacement. Inflammatory myositis was less likely given her improvement without additional steroids or immunosuppression.
After two months of weight-based levothyroxine, her TSH normalized to 4.3 mIU/L (RR: 0.41–4.81 mIU/L), T4 to 7.5 ug/dL (RR: 5–10.69 ug/dL), free T4 to 2.3 ng/dL (RR: 1.1–2.4 ng/dL), free T3 to 103.7 ng/dL (RR: 78–158 ng/dL) and CK to 82 U/L (RR: 40–308 U/L).
Discussion
IRAEs typically develop within weeks to months after immune checkpoint inhibitor treatment initiation and can affect multiple organ systems.1,2 CTLA-4 acts in lymphoid organs by competing with CD28 binding to CD80/86, the co-stimulatory signal necessary for T cell activation. PD-1 inhibits effector T cells at later stages of the immune response in peripheral tissues.5,6 Patients treated with anti-CTLA-4 and anti-PD-1/PD-1L therapy have distinct IRAEs, with anti-CTLA-4-related IRAE being generally more severe.
The exact mechanisms underlying IRAEs remain elusive. Potential underlying mechanisms resulting in IRAEs include increased T cell activity against antigens that are shared by tumor and healthy tissues, increased levels of preexisting autoantibodies, increased levels of inflammatory cytokines and enhanced complement-mediated inflammation due to direct binding of an anti-CTLA-4 antibody to CTLA-4 expressed on normal tissues, such as the pituitary.1
Endocrinopathies related to immune checkpoint blockade can affect the pituitary gland (e.g., hypophysitis, corticotrophin decrease, secondary adrenal insufficiency), thyroid (e.g., hyperthyroidism, hypothyroidism, thyroiditis), adrenal glands (e.g., primary adrenal insufficiency) and pancreas (e.g., diabetes mellitus).7 Hypophysitis is more commonly reported with anti-CTLA-4 therapy, probably due to anti-CTLA-4 binding to CTLA-4, which is normally expressed in pituitary cells.8
Thyroid dysfunction has been reported in up to 10% of patients treated with PD-1/PD-L1 blockade. In a cohort of patients with non-small-cell lung cancer treated with pembrolizumab (a PD-1 inhibitor), investigators found hypothyroidism was often preceded by a transient and asymptomatic period of hyperthyroidism. Anti-thyroid antibodies were highly and temporally associated with nearly all cases of thyroid dysfunction, suggesting PD-1 inhibitors may unmask latent autoimmunity.9 Our patient had preexisting thyroid antibodies and experienced transient hyperthyroidism prior to developing hypothyroidism and myositis, as evidenced by myalgias, muscle weakness and elevated CK levels.
Hypothyroid myopathy can mimic inflammatory myopathies and should be considered in patients treated with immune checkpoint blockade (ICB) because IRAE endocrinopathies are more common than inflammatory myositis. Patients with frank hypothyroidism have a wide spectrum of muscular symptoms, including stiffness, myalgias, cramps and easy fatigability.10 Elevated serum CK is found in 37–60% patients with hypothyroidism but does not correlate with the severity of myopathic symptoms.
Elevated CK levels (greater than 10 times above the upper limit of normal) may suggest rhabdomyolysis, which is a rare complication of hypothyroidism. Cases of proximal myopathy with a presentation similar to polymyositis involving the shoulders and hips and with an associated CK elevation have been reported.11 Muscle biopsy in hypothyroid myopathy can be normal and can have nonspecific changes, such as type II fiber atrophy. The treatment of choice in hypothyroid myopathy is thyroid hormone replacement.10
Although myalgias and muscle weakness have been reported in clinical trials of participants on immunotherapy, the etiology of these symptoms remains unclear. Immunotherapy-related inflammatory myopathies have been reported.3 In a case series of 654 oncological patients receiving PD-1 inhibitors, five developed myopathy (0.76%), all occurring in patients treated with pembrolizumab.12 Patients developed weakness after a median of two cycles of pembrolizumab treatment and shared common clinical features of oculobulbar and neck flexor weakness. One patient had concomitant acetylcholine receptor (AChR) antibody positive myasthenia gravis. Pathologic findings were varied, and the two patients with necrotizing myopathy had fatal outcomes despite treatment with high-dose steroids and plasma exchange. Interestingly, CK levels did not correlate with disease severity, because the patient with the highest
CK level had a relatively benign
clinical course.12
Physicians in many specialties (including rheumatology) are becoming more aware of IRAEs and creating guidelines for their evaluation and management. Current recommendations for patients with suspected myositis include manual strength testing, dynamometry, blood work (including serologies for CK, aldolase, ESR, CRP, ANA and a myositis panel (e.g., Jo-1, PL-7, PL-12, EJ, OJ, Mi-2, SRP), electromyography and MRI of the affected muscle.3
Because endocrinopathies are relatively common IRAEs and patients with hypothyroidism often have abnormal CK levels, it may prove useful to check thyroid function tests in evaluating patients on immunotherapy who present with myositis.
 Shuwei Wang, MD, completed her rheumatology fellowship in the Division of Rheumatology at Columbia University Irving Medical Center, New York City. She is now an attending rheumatologist at Morristown Medical Center, N.J.
Shuwei Wang, MD, completed her rheumatology fellowship in the Division of Rheumatology at Columbia University Irving Medical Center, New York City. She is now an attending rheumatologist at Morristown Medical Center, N.J.
 Gulam A. Manji, MD, PhD, is an assistant professor of medicine in the Medical Oncology Division at Columbia University Irving Medical Center.
Gulam A. Manji, MD, PhD, is an assistant professor of medicine in the Medical Oncology Division at Columbia University Irving Medical Center.
 Anca D. Askanase, MD, MPH, is an associate professor of medicine in the Division of Rheumatology and director of the Lupus Center at Columbia University Irving Medical Center.
Anca D. Askanase, MD, MPH, is an associate professor of medicine in the Division of Rheumatology and director of the Lupus Center at Columbia University Irving Medical Center.
References
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018 Jan 11;378:158–168.
- June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017 May 5;23(5):540–547.
- Cappelli LC, Shah AA, Bingham CO 3rd. Immune-related adverse effects of cancer immunotherapy—implications for rheumatology. Rheum Dis Clin North Am. 2017 Feb;43(1):65–78.
- Shenoy N, Esplin B, Barbosa N, et al. Pembrolizumab induced severe sclerodermoid reaction. Ann Oncol. 2017 Feb 1; 28(2):432–433.
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002 Aug;8(8):793–800.
- Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016 Nov 3;375:1767–1778.
- Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017 Jul;58:70–76.
- Iwama S, Deremigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014 Apr 2;6(230):230ra45.
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017 Mar 1;28(3):583–589.
- Sindoni A, Rodolico C, Pappalardo MA, et al. Hypothyroid myopathy: A peculiar clinical presentation of thyroid failure. Review of the literature. Rev Endocr Metab Disord. 2016 Dec;17(4):499–519.
- Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with strikingly elevated serum creatine kinase level. Muscle Nerve. 2002 Jul;26(1):141–144.
- Liewluck T, Kao JC, Mauermann ML. PD-1 inhibitor associated myopathies: Emerging immune-mediated myopathies. J Immunother. 2018 May;41(4):208–211.