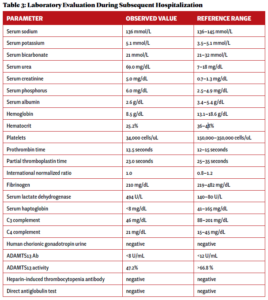
Two weeks after initial hospital discharge, the patient returned to an emergency department complaining of worsening abdominal and bilateral flank pain. Additional imaging was ultimately negative for new thrombosis or infarction, and her symptoms were attributed to volume overload in the setting of dialysis. Laboratory testing incidentally revealed new-onset thrombocytopenia and low-grade schistocytes (see Table 3).
These laboratory findings and her clinical presentation were ultimately consistent with microangiopathy hemolytic anemia and thrombocytopenia, collectively a thrombotic microangiopathy syndrome. In the setting of low complement levels with no additional features of an overlap rheumatic syndrome, a diagnosis of c-TMA was made.
Evaluation was without findings for additional etiologies in the differential diagnosis for a TMA syndrome, including thrombotic thrombocytopenic purpura (TTP); heparin-induced thrombocytopenia; hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome; eclampsia; drug-induced TMA; scleroderma renal crisis; antiphospholipid antibody syndrome; malignant hypertension; Shiga toxin-producing Escherichia coli infection; and malignancy.
Anti-complement therapy and plasmapheresis were initially considered, but the patient’s platelets continued to increase along with a slow resolution of the c-TMA syndrome without additional intervention. Further escalation of immunosuppressive therapy was, therefore, deferred.
The patient was ultimately discharged five days later and continued on a prolonged, moderate-dose corticosteroid taper. Two weeks later, laboratory evidence indicated complete resolution of the c-TMA syndrome.
Discussion
ANCA-associated vasculitis is a rare autoimmune condition characterized by inflammation of small and medium-sized vessels due to antibodies to either proteinase 3 or MPO. Subtypes of ANCA-associated vasculitis include microscopic polyangiitis (MPA), granulomatous polyangiitis (GPA), and eosinophilic granulomatous polyangiitis (EGPA). Multi-organ dysfunction, including renal and respiratory impairment, is a typical feature of ANCA-associated vasculitis.1
TMA is characterized by microangiopathy hemolytic anemia and thrombocytopenia, which can also demonstrate varying degrees of multi-organ dysfunction resembling ANCA-associated vasculitis. c-TMA, also known as atypical hemolytic uremic syndrome (aHUS), is a subtype of TMA. The pathogenesis of c-TMA is secondary to either a hereditary or acquired dysfunction of proteins, resulting in dysregulation of the alternative pathway of the complement cascade.2 The excessive activation of the complement cascade results in uncontrolled damage to vascular endothelium, causing the hypocomplementemia and ischemic renal injury that are hallmarks of its clinical presentation.3
c-TMA is ultimately a clinical diagnosis of exclusion. In the initial evaluation of a suspected TMA syndrome, it is essential to rule out primary TMA syndromes (including TTP and aHUS) and evaluate for systemic disease as a cause of secondary TMA (see Table 4).