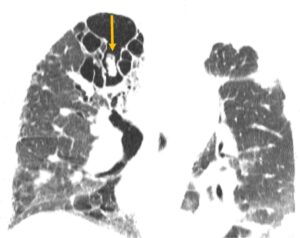
Figure 1
CT of the chest with mycetoma (arrow)
Joint and bone pain are among the most common reasons for rheumatology consultations. A thorough differential diagnosis is essential for rheumatologists to distinguish the inflammatory pain of such conditions as rheumatoid arthritis from other causes because management strategies can vary significantly. Bones are metabolically active organs influenced by multiple factors, including trace elements, such as fluoride. Fluoride, the ionic form of fluorine, is required in trace amounts for the normal development of bones and teeth.
The inorganic component of the bone extracellular matrix primarily consists of hydroxyapatite. In mineralized tissues, fluorine is incorporated into apatite crystals through ion exchange, forming fluorapatite. This incorporation increases bone density and makes the matrix more resistant to resorption. However, excessive deposition of fluorapatite can lead to increased osteoblasticfor transplants requiring antifungal activity and disorganized osteoblastic prophylaxis, a new form of fluoride reactions, leading to a condition known as skeletal fluorosis.9 3,6,7,11
Skeletal fluorosis from excess fluoride intake is characterized by disorganized osteoblastic and osteoclastic activity, which can result in severe bone and joint pain, skeletal deformities and fractures, often mimicking rheumatic diseases.4 Bone changes observed in skeletal fluorosis include osteosclerosis, osteomalacia, osteoporosis, exostosis formation and periostitis. The severity of the disease often correlates with the individual’s total fluoride intake. Traditionally, skeletal fluorosis has occurred in individuals with high environmental exposure to fluoride.6-8 However, with advancements in medical therapies, such as the U.S. Food & Drug Administration’s approval of second-generation antifungal medications in 2002 and the increased need for transplants requiring antifungal prophylaxis, a new form of fluoride exposure is becoming more prevalent.9
Case Presentation
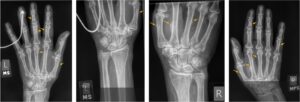
Figure 2
Hand X-rays
A 70-year-old woman with a history of idiopathic pulmonary fibrosis (IPF) status a year following a single, left lung transplant; type 2 diabetes; and osteoarthritis, was admitted for hypoxemic respiratory failure, left lung consolidations and worsening severe joint pain. Approximately a month before this presentation, she had been admitted for pneumonia—treated with antibiotics and 60 mg of glucocorticoids daily— and volume overload.
She was discharged on a steroid taper with stable pulmonary function tests, but no changes to chest X-rays. Her lung transplant course had been complicated by progressive dyspnea and concerns for chronic lung allograft dysfunction and infection. A previous biopsy of the transplanted lung did not show signs of rejection. Past bronchoalveolar lavage had grown five colonies of scedosporium/pseudallescheria boydii.
At baseline, the patient required 4 L of oxygen/minute at rest and 6 L with exertion. Her current home medications included 10 mg of prednisone daily (tapered to 10 mg daily), nintedanib, tacrolimus, mycophenolate, acyclovir, azithromycin, voriconazole, trimethoprim/sulfamethoxazole, metoprolol, atorvastatin, insulin, levothyroxine and gabapentin. A rheumatologist was consulted for severe, worsening and debilitating pain that was refractory to corticosteroid therapy.
On evaluation, the patient reported increased bone pain for the last three months, particularly in her hands, back, arms, legs and shoulders. She denied joint swelling or warmth, but noted that heat packs offered some relief. The pain was described as constant, and she was unable to make a fist or complete her activities of daily living due to severe pain. Her vitals were stable and within normal limits on four liters of oxygen via nasal cannula.
The physical exam revealed diffuse coarse crackles throughout both lung fields, full range of motion in all joints when moved slowly, no evidence of synovitis and diffuse tenderness in the arms, shins, hands, forearms and ribs with moderate pressure palpation. Initial lab results were overall reassuring, with a normal complete blood count (CBC), erythrocyte sedimentation rate (ESR), parathyroid hormone (PTH), 25-hydroxyvitamin D, tacrolimus trough and muscle enzymes. Rheumatoid factor (RF) and cyclic citrullinated peptide (CCP) antibody levels were previously checked and found to be within normal ranges. Aside from an abnormal blood gas (stable from prior), the only other abnormal finding was an elevated alkaline phosphatase (ALP) level at 203 U/L (reference range: 35–105 U/L).
Based on the patient’s history, physical examination, elevated ALP and X-ray findings, voriconazole-induced periostitis (VIP) was diagnosed as the cause of her pain. This diagnosis was further confirmed when her fluoride level returned at greater than four times the upper limit of normal.
An extensive infectious evaluation revealed a positive plasma Mucorales polymerase chain reaction (PCR). Consequently, her treatment was switched from voriconazole to amphotericin, resulting in prompt improvement in her bone pain and normalization of her ALP levels (see Figure 4, below).
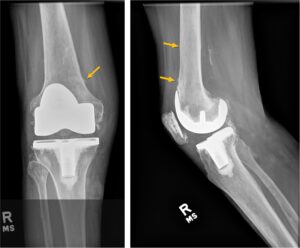
Figure 3
Right knee X-ray
Discussion
VIP is a form of skeletal fluorosis most commonly seen in patients receiving high doses and/or prolonged courses of voriconazole. Voriconazole is widely used for prophylaxis of fungal infections post-transplant and for the treatment of invasive aspergillosis.1,3,14
Excess fluoride can lead to disorganized bone formation and findings of periostitis.1 The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine lists the upper limit of fluoride from all sources in adults as 10 mg, based on higher levels being associated with dental and skeletal fluorosis.12 Voriconazole, a fluorinated antifungal agent, contains three fluorine atoms, compared with two in other second-generation triazole antifungal medications (e.g., fluconazole, posaconazole, isavuconazole). It has excellent oral bioavailability at 96%. It is believed that the unbound form of the drug affects bone, and 42% of the drug remains unbound by plasma proteins.3,4 Gerber et al. assessed 43 patients on triazole antifungal medication (20 of whom were taking voriconazole) and found that bone pain and radiologic evidence of periostitis were exclusively seen in patients receiving long-term voriconazole.11 This is likely because agents like posaconazole and isavuconazole, while fluorinated, do not metabolize to the free fluoride form in the body.5
A daily dose of 400 mg of voriconazole provides approximately 65 mg of fluoride, far exceeding the upper limit of safety. However, even with elevated blood fluoride levels, most individuals on voriconazole do not develop symptoms of skeletal fluorosis or periostitis, suggesting factors beyond fluoride exposure mediate VIP.
Estimates of the incidence of periostitis in those treated with voriconazole range from 15% to 50%.3,4,11 In affected individuals, periostitis and exostoses typically develop after six or more months of voriconazole therapy, although the range is variable. This variability likely reflects individual differences in past fluoride exposure, pharmacokinetics and renal clearance. The kidneys excrete 60% of daily ingested fluoride in persons with normal renal function. Higher daily and cumulative doses of voriconazole are positively correlated with the development of periostitis.3-5,11,12
Ashmeik et al. examined nine patients with VIP and 122 patients without, finding that a 31.5 g increase in cumulative voriconazole dose was associated with 8% higher odds of incident periostitis, and increased treatment duration (63 days) was associated with 7% higher odds of periostitis.15
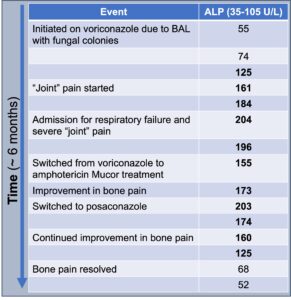
Figure 4
ALP trends over time
Clinically, symptoms of VIP include diffuse, often severe, and constant bone pain that worsens with palpation. In severe cases of periostitis, palpable bony overgrowth and nodules can occur. Joint swelling has been reported, as have non-specific symptoms of myalgias and weakness. The pain tends to be minimally responsive or unresponsive to steroids, colchicine, and opiates. Although any bone can be affected, the ribs, proximal long bones, shoulders and hands are most commonly involved. Excess fluoride can also deposit on dental enamel, resulting in dental fluorosis characterized by white streaks or specks.3,4
Other conditions to consider in a patient with diffuse bone pain include malignancy (particularly leukemia and lymphoma), bone metastasis, multi-focal avascular necrosis, autoimmune inflammatory rheumatic diseases, such as polymyalgia rheumatica, rheumatoid arthritis, osteoarthritis, calcineurin inhibitor-induced pain syndrome and primary pain syndromes, such as fibromyalgia.
The differential diagnosis for periostitis and/or enthesopathic changes includes fluorosis; autoinflammatory bone diseases, such as chronic non- bacterial osteomyelitis; HLA-B27- associated conditions, such as spondyloarthritis or psoriatic arthritis; trauma; infection, such as congenital syphilis; hypertrophic osteoarthropathy; venous stasis-related periosteal reaction; thyroid acropachy; and hypervitaminosis A.2,4,13
Diagnosis of VIP should include: 1) typical symptoms, such as subacute, often severe musculoskeletal pain; 2) compatible radiographic findings on X-ray, computed tomography or bone scan; 3) absence of an alternative diagnosis; and 4) improvement after drug cessation.
Specific laboratory findings can strengthen the diagnosis of VIP. New periosteal bone growth in VIP leads to elevations in ALP and other markers of bone remodeling. Ashmeik et al. found that in patients on voriconazole, an increased average ALP (50 IU/L) was associated with higher odds of incident VIP (odds ratio 1.34 [95% confidence interval 1.11, 1.61], P = 0.002).15 Elevated serum fluoride levels often correlate with symptoms and periostitis, although they are not always present.
Calcium, phosphate, PTH, 25-hydroxy-vitamin D and trough concentrations of voriconazole are typically within normal limits.1,3,4 A retrospective study by Moon et al. found that in patients taking voriconazole who underwent a bone scan and plasma fluoride measurement for skeletal pain, elevated fluoride and ALP levels strongly correlated with periostitis on imaging compared to those without periostitis on imaging (fluoride 12.78 ± 0.96 vs. 3.61 ± 1.29 μmol/L; P < 0.001; ALP 273 ± 35.6 vs. 117 ± 15.7; P = 0.02).14
Radiographic findings of VIP and skeletal fluorosis typically include periosteal thickening, calcification of ligaments, and osteosclerosis. These findings are well-demonstrated on X-ray and bone scan. While magnetic resonance imaging (MRI), CT, single photon emission computed tomography (SPECT/CT), and fluorodeoxyglucose (FDG) positron emission tomography (PET) scans can show abnormalities, these more expensive modalities are generally unnecessary for diagnosing VIP.3,4,11
Periostitis manifests as multifocal periosteal thickening with dense, irregular, nodular, and usually bilateral new bone formation. Enthesopathic changes in affected long bones are common, sometimes with smooth or asymmetric periosteal reactions.2,4
X-ray imaging reveals multifocal periostitis with periosteal thickening and reaction, showing new bone formation that can be nodular or fluffy. Osteoblastic lesions, periosteal and ligament calcifications, osteosclerosis and entheseal ossification are commonly reported. More rare X-ray findings include bony exostoses at multiple sites, interosseous membrane calcification, metadiaphyseal widening and sacroiliac joint involvement. Isotope bone scans demonstrate increased tracer uptake in affected bones, typically showing fluffy or lamellar periosteal reactions. MRI may reveal periosteal edema.2,10
Discontinuation, and occasionally dose reduction, of voriconazole is essential for improving pain symptoms, representing the only curative treatment for VIP.
Gerber et al. reported clinical improvement in all cases of VIP upon ceasing voriconazole.11
Pain should improve with medication discontinuation, with some patients experiencing relief within days and most seeing resolution within two to four months. ALP and radiologic abnormalities typically normalize within three to four months.4,5,10 However, elevated blood fluoride levels may persist for an extended period, reflecting fluoride sequestration in, and slow release from, the skeleton.1
Supportive care includes pain management and addressing other conditions that may impact bone health such as hyperparathyroidism or malnutrition if present. Some less studied strategies, such as urinary alkalinization for pH-dependent fluoride excretion, have been suggested but have yet to be widely studied or used.
Conclusion
The patient described in this case exhibited classic findings of VIP, including diffuse bone pain, multifocal periosteal reactions on X-rays, enthesopathy, calcific tendinitis, elevated ALP levels and high fluoride levels. Notably, the patient’s symptoms and elevated ALP levels quickly resolved following the discontinuation of voriconazole. This case underscores the importance of rapid recognition and diagnosis of VIP by rheumatologists, which can prevent unnecessary and costly invasive procedures and significantly enhance the patient’s quality of life.
Amanda Moyer, MD, is a fourth-year fellow in a combined adult and pediatric rheumatology fellowship at Stanford School of Medicine, Palo Alto, Calif.
References
- Rad B, Saleem M, Grant S, et al. Fluorosis and periostitis deformans as complications of prolonged voriconazole treatment. Ann Clin Biochem. 2015 Sep;52(Pt 5):611–614.
- Newman C, Sharma R, Silverstone L, et al. Voriconazole-induced periostitis. Radiopaedia. org. 2022 Jun 14. Revised 2024 Apr 2. https:// radiopaedia.org/articles/147153.
- Guarascio AJ, Bhanot N, Min Z. Voriconazole- associated periostitis: Pathophysiology, risk factors, clinical manifestations, diagnosis, and management. World J Transplant. 2021 Sep;11(9):356–371.
- Tan I, Lomasney L, Stacy GS, et al. Spectrum of voriconazole-induced periostitis with review of the differential diagnosis. AJR Am J Roentgenol. 2019 Jan;212(1):157–165.
- Bennett MJ, Balcerek MI, Lewis EA, et al. Voriconazole-associated periostitis: New insights into pathophysiology and management. JBMR Plus. 2021 Oct 6;6(2):e10557.
- Lindsay R. Fluoride and bone—quantity versus quality. N Engl J Med. 1990 Mar;322(12):845–846.
- Ciosek Ż, Kot K, Kosik-Bogacka D, et al. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules. 2021 Mar;11(4):506.
- Dhar V, Bhatnagar M. Physiology and toxicity of fluoride. Indian J Dent Res. 2009 Jul–Sep;20(3):350–355.
- Reber JD, McKenzie GA, Broski SM. Voriconazole-induced periostitis: Beyond post-transplant patients. Skeletal Radiol. 2016 Jun;45(6):839–842.
- Adwan MH. Voriconazole-induced periostitis: A new rheumatic disorder. Clin Rheumatol. 2017 Mar;36(3):609–615.
- Gerber B, Guggenberger R, Fasler D, et al. Reversible skeletal disease and high fluoride serum levels in hematologic patients receiving voriconazole. Blood. 2012 Sep;120(12):2390–2394.
- Office of Dietary Supplements. Fluoride fact sheet for health professionals. National Institutes of Health. 2024 Jun 26. https://ods.od.nih.gov/ factsheets/Fluoride-HealthProfessional.
- Rana RS, Wu JS, Eisenberg RL. Periosteal reaction. AJR Am J Roentgenol. 2009 Oct;193(4):W259–272.
- Moon WJ, Scheller EL, Suneja A, et al. Plasma fluoride level as a predictor of voriconazole- induced periostitis in patients with skeletal pain. Clin Infect Dis. 2014 Nov 1;59(9):1237–1245.
- Ashmeik W, Schirò S, Joseph GB, et al. Associations of cumulative voriconazole dose, treatment duration, and alkaline phosphatase with voriconazole-induced periostitis. Skeletal Radiol. 2024 May 17.
