Rheumatologists often rely on magnetic resonance imaging (MRI) in the evaluation of suspected muscular diseases. Here, we describe a case in which unexpected findings on MRI pointed to a diagnosis rarely considered as a mimicker of rheumatologic disease.
The Case
A 19-year-old man of Middle Eastern descent was admitted to our hospital for evaluation of progressive fatigue and muscle weakness. He had been a competitive 5- and 10-kilometer runner until five months before, when he started noticing muscle weakness and wasting. He reported leg pain during and after running. He continued to run for exercise, but decreased his pace due to leg pain and shortness of breath. During this period, he also lost 20 lbs., going from a slim but muscular build to a wasted appearance. He developed cold intolerance and described color changes in his fingers and hands with cold exposure. He reported intermittent hematuria after running and dark stools suspicious for melena. He denied diarrhea. He stated his appetite was normal and that he was eating a well-rounded diet, which his mother confirmed. He had decreased libido and had noticed his testicles had become smaller. He denied any rash or patchy alopecia but noted diffuse mild hair loss. He denied oral ulcers and photosensitivity.
Prior to admission, he had been evaluated by a nephrologist, who diagnosed recurrent fluid-responsive acute kidney injury, anemia and low testosterone. His medications were oral iron supplements and diphenhydramine as needed for sleep. He had no allergies. His family history was notable for consanguinity, as his parents are distant cousins. His father had thyroid cancer, and a great aunt had multiple sclerosis. There was no family history of muscular dystrophy, myositis, systemic lupus erythematosus or scleroderma. The patient had recently relocated from the Middle East to Boston to enroll in college. He drank one glass of wine per week but denied tobacco or illicit drug use.
On physical exam, he was afebrile, with a peak temperature of 98.1ºF during the previous 24 hours. His heart rate was 49, and his blood pressure was 101/66. He was breathing at a rate of 18 breaths per minute, and he had an oxygen saturation of 100% on room air. He was slim and had a body mass index of 17. He appeared somewhat listless. He was somewhat reluctant to put away his math homework for the exam, but was cooperative overall. His sclera were clear, and no heliotrope rash or periorbital edema was present. He did not have oral or nasal ulcers, swelling or tenderness to palpation of his salivary glands, or cervical or supraclavicular lymphadenopathy. His lungs were clear to auscultation. His heart rate was bradycardic but regular, without murmurs. His abdomen was notable for well-developed abdominal muscles. There was no hepatosplenomegaly. His extremities were warm and non-edematous. He had strong radial and pedal pulses. Neurological exam revealed good comprehension and intact cranial nerves. Muscle strength was 5-/5 in the proximal lower-extremity muscles, and 5/5 for all other muscle groups. There were no sensory deficits. His gait was normal. Joint exam was unremarkable. Skin exam revealed dry skin and erythema of the interdigital webs of his hands. He also had signs of chronic excoriation of the distal fingers of his right hand.
Labs on admission were notable for a creatinine of 1.46 mg/dL, which corrected to 0.9 mg/dL with hydration. His hematocrit was 33.4%, down from 40.6% two weeks prior. His reticulocyte index was 0.6%. His alanine transaminase was mildly elevated at 53 units/L. Albumin was 4.1 g/dL. His white blood cell count was 3,400/uL, and his platelet count was 159,000/uL. Urinalysis earlier in the month had 2+ blood, but was negative for blood or protein on admission. C-reactive protein was 0.2 mg/L (0–3 mg/L), and erythrocyte sedimentation rate was 7 mm/hr (0–12 mm/hr). Viral hepatitis and HIV serologies were negative. Creatinine kinase (CK) had been 229 units/L three weeks prior to admission, but had normalized.
Anorexia nervosa can present with a number of symptoms, signs & even lab results that can initially appear consistent with a rheumatologic disorder, such as limb pain, weakness, hair loss, rashes, cytopenias & pathologic fractures.
We considered a number of different etiologies for the underlying process. Inflammatory myositis, such as polymyositis, dermatomyositis and immune-mediated necrotizing myopathies, seemed unlikely because these conditions are usually painless and feature elevated CK and inflammatory markers. Rheumatoid factor, antinuclear antibodies (ANA), anti-dsDNA, antineutrophil cytoplasmic antibodies (ANCA), and anti-glomerular basement membrane (anti-GBM) antibodies had been checked previously and were all within normal ranges. A kidney biopsy performed for hematuria one month previously revealed normal kidney parenchyma.
We next considered non-inflammatory myopathies, some of which can present with normal CK. A number of endocrine dyscrasias can lead to myopathies, including hypo- and hyperthyroidism, hyperparathyroidism, vitamin D deficiency, Cushing’s disease and pituitary disorders. Thyroid tests were normal. His 25-OH vitamin D was 37 ng/mL. His total testosterone level was 13.5 ng/dL (normal 249–836 ng/dL). Prolactin, follicle-stimulating hormone and luteinizing hormone levels were normal. A random cortisol level was elevated at 25.9 (2.3-11.9 ug/dL), but a morning cortisol level was normal at 14.6 ug/dL (6.2–19.4 ug/dL). A pituitary MRI was normal.
We also considered hereditary muscle disorders. Myopathy associated with mutations in the GNE gene was particularly interesting, as it often presents in early adulthood and has a higher prevalence among patients of Middle Eastern descent. However, it usually presents with distal lower extremity weakness that then progresses proximally. An MRI shows fatty replacement of the muscle. Glycogen and lipid storage diseases were also considered. Among these, McArdle’s disease, which is caused by myophosphorylase deficiency, was especially interesting, as it often presents with exercise-induced myalgia and fatigue. It can cause transient myoglobinuria and acute kidney injury due to rhabdomyolysis. We also considered carnitine-palmitoyl transferase 2 deficiency, which can present in the teens and early 20s with cramps, myalgia, myoglobinuria and exercise intolerance.
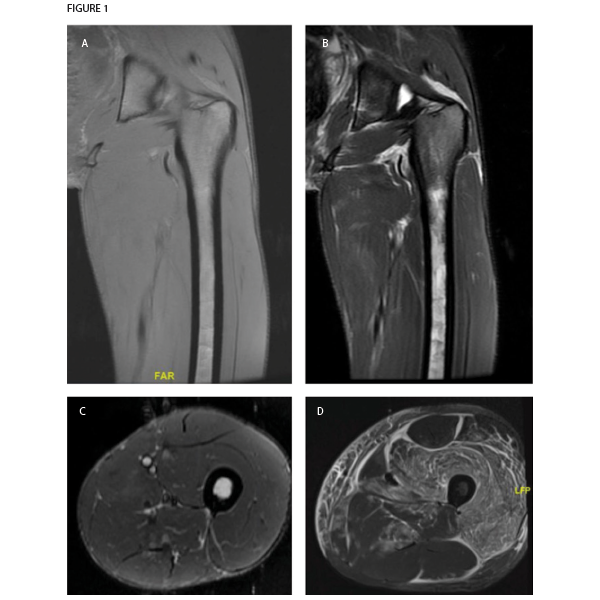
Figure 1. Magnetic resonance imaging of patient’s left thigh. Coronal sections of (A) T1-weighted and (B) T2-weighted sequences both demonstrate extremely thin layer of subcutaneous fat, also seen in the (C) axial section section of STIR sequence imaging. The muscle parenchyma does not have any regions of high T2 signal intensity to suggest muscle edema, as is seen in an axial STIR image from a representative patient with dermatomyositis (image courtesy of Simon Helfgott, MD) (D). The patient’s bone marrow has low T1 and high T2 and STIR signals in the diaphysis of the femur, consistent with serous atrophy. Normal bone marrow has high T1 signal and low T2 and STIR signals.
Our list of differential diagnoses also included occult malignancy, especially in light of the pancytopenia. Lactate dehydrogenase was 182 units/L. The patient refused a bone marrow biopsy. Recent upper and lower endoscopy results were normal. Computed tomography (CT) of the chest, abdomen and pelvis did not suggest malignancy, but was notable for diffuse abnormal appearance of the muscles of the chest, potentially consistent with myositis or myopathy.
An MRI of the thigh was obtained to further characterize the muscle abnormality initially seen in the CT scan. Notably, the muscle parenchyma itself was completely normal, with no signs of edema or fatty replacement. Instead, the MRI revealed a marked decrease in subcutaneous fat. Also, T2 signal hyperintensity was present in the bone marrow of the femur, consistent with serous atrophy. No fractures were seen.
Serous atrophy or gelatinous replacement of the bone marrow is typically seen in situations of starvation, such as cachexia due to malignancy. In younger individuals, it is often caused by anorexia nervosa.1 On MRI, the bone marrow is hypointense on T1-weighted imaging and hyperintense on T2 and STIR sequences, similar to fluid. This unusual pattern of signals is sometimes misinterpreted as a technical problem with the MRI machine, and it is not uncommon for patients to have a second MRI before the diagnosis is made. It does not enhance significantly with contrast, which would instead indicate neoplastic replacement of bone marrow. Patients with serous atrophy of the bone marrow are at high risk of stress fractures (nearly half of patients in one cohort), which can be difficult to detect on MRI due to the hyperintense bone marrow signal.1
When these MRI findings were presented to the patient, he admitted to behavior consistent with an eating disorder, including both deliberate starvation and vigorous exercise. He was referred to psychiatry for treatment.
Discussion
Anorexia nervosa is an eating disorder that predominantly occurs in young women, but can also occur in men. Anorexia nervosa can present with a number of symptoms, signs and even lab results that initially appear consistent with a rheumatologic disorder, including limb pain, weakness, hair loss, rashes, cytopenias and pathologic fractures. For example, leukopenia, anemia and chilblains in a young woman may lead to a referral to rheumatology for consideration of systemic lupus erythematosus. Anorexia nervosa can also cause muscle and bone disease that may prompt evaluation by a rheumatologist.
Muscle disease in anorexia nervosa is seen mainly in cases of severe weight loss. Initially, patients lose mostly adipose tissue, but with progressive weight loss, muscle mass is also affected, leading to proximal muscle weakness and, in some cases, muscle pain. Limb pain, particularly leg or foot pain, can also be due to stress fractures, as discussed below. Symptoms of neuropathy, including absent reflexes, may also be present.2 Elevated CK levels are rare, but rhabdomyolysis can occur if hypophosphatemia is present and not treated prior to refeeding. Electromyography may show myopathy and/or neuropathy. Unlike dermatomyositis and polymyositis, muscle edema on MRI is absent. Muscle biopsies of patients with profound weight loss due to anorexia nervosa characteristically demonstrate selective atrophy of type II (i.e., fast twitch) muscle fibers without inflammatory infiltrates.2,3
Osteopenia, osteoporosis and abnormalities of hip architecture can occur in both male and female patients with anorexia nervosa, usually correlating with amenorrhea in female patients and profoundly low testosterone in male patients.4 Other hormonal axes, such as growth hormone, adipokine and insulin pathways, may also cause low bone density in patients with anorexia nervosa. Stress fractures, especially of the lower limbs, can occur. The risk of stress fractures is increased not only by low bone density but likely also by serous atrophy, which is thought to provide less mechanical support to the surrounding bone during impact compared to normal cellular bone marrow.1
Treatment
Treatment of anorexia nervosa includes psychiatric treatment and refeeding, which reverses serous atrophy and many of the other changes discussed above. Unfortunately, bone density rarely returns to normal.4
 Anna Helena Jonsson, MD, PhD, is a senior rheumatology fellow at Brigham and Women’s Hospital in Boston.
Anna Helena Jonsson, MD, PhD, is a senior rheumatology fellow at Brigham and Women’s Hospital in Boston.
 Julia F. Charles, MD, PhD, is an assistant professor in orthopedics and rheumatology at Brigham and Women’s Hospital.
Julia F. Charles, MD, PhD, is an assistant professor in orthopedics and rheumatology at Brigham and Women’s Hospital.
References
- Boutin RD, White LM, Laor T, et al. MRI findings of serous atrophy of bone marrow and associated complications. Eur Radiol. 2015;25(9):2771–2778.
- McLoughlin DM, Wassif WS, Morton J, et al. Metabolic abnormalities associated with skeletal myopathy in severe anorexia nervosa. Nutrition. 2000;16(3):192–196.
- McLoughlin DM, Spargo E, Wassif WS, et al. Structural and functional changes in skeletal muscle in anorexia nervosa. Acta Neuropathol. 1998;95(6):632–640.
- Misra M, Klibanski A. Anorexia nervosa and bone. J Endocrinol. 2014;221(3):R163–R176.


