Cellular therapy refers to the use of live cells to replace or repair a damaged organ system. The first widespread use of this approach occurred more than 50 years ago when hematopoietic stem cells (HSCs) from the bone marrow of a healthy donor (allogeneic) were used to replace the hematopoietic system of a recipient after it was ablated during chemo/radio therapy of leukemia, the recipient’s hematopoietic system being “collateral damage” during the eradication of the unwanted leukemia cells.
This approach was later extended to the use of autologous stem cells in various malignant conditions such as breast cancer, allowing the use of more intense chemotherapy. In this context, the therapy is referred to as “hematopoietic stem cell support.” This principle was adopted for treating severe autoimmune disease more than 10 years ago in patients who failed to respond to conventional immunosuppression but in whom the marrow toxicity restricted dose escalation. Over 1,000 autoimmune disease patients have been treated, with around 30% achieving sustained remission.
Recently another cell, the mesenchymal stem cell (MSC), has been applied to the treatment of autoimmune disease. The concept is completely different from HSC in that pretreatment with chemotherapy is not required, and the MSCs are thought to home in on damaged tissue and exert a local paracrine healing effect. This article will review the basis of this novel treatment and results of recent trials.
Adult Stem Cells
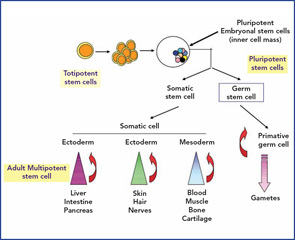
A typical adult stem cell is one which, on division, results in one daughter cell that can further differentiate and replenish a whole compartment or tissue, while the other remains fully “stem” and self-renewing. This is called asymmetric division, and the stem cell is known as multipotent. (See Figure 1, p. 21.)
The best-studied example of this type of adult stem cell is the hematopoietic stem cell, which produces a trillion differentiated blood cells per day for the life of the animal. This remarkable property is possible because of a complex interaction between the stem cell and other cells and structures within the bone marrow microenvironment called the niche. However, other stem cells exist in the embryo from the moment of conception; the first eight to 16 divisions produce totipotent stem cells by symmetrical division. The word totipotent is used here because each cell may produce a full individual animal because the placental elements are present. Identical twins are the best example. Once the blastocyst has formed with an inner cell mass (destined to become the fetus) and an outer membrane (the early placenta), then the embryonal stem cells (ESCs) from the inner cell mass are called pluripotent. This means that all organs and tissues may be produced but no longer an intact individual. (See Figure 2, p. 22.)
Recently, another cell, the mesenchymal stem cell (MSC), has been applied to the treatment of AD. The concept is completely different from HSC in that pretreatment with chemotherapy is not required, and the MSCs are thought to home in on damaged tissue and exert a local paracrine healing effect.
ESCs have extensive plasticity in terms of potential in vitro tissue differentiation, but they also carry an intrinsic risk of developing teratomas. In addition, the harvesting of pluripotent ESC requires destruction of an embryo, which raises ethical and theological issues limiting their utility.
The use of adult stem cells in treatment carries neither ethical nor teratoma complications, but these cells are restricted in their cross-tissue differentiation potential. Although claims of adult stem cell plasticity abound, the number of such transdifferentiation events is too small for a meaningful biological effect. What is emerging, however, is the potential positive paracrine effects of infused adult stem cells such as MSCs on damaged tissue such as myocardium (e.g., postinfarct or inflamed organs, such as acute graft-versus-host disease [GvHD]).
The first use of adult stem cells in clinical medicine was that of hematopoietic stem cells in the treatment of malignant disorders.
Hematopoietic Stem Cell Transplantation
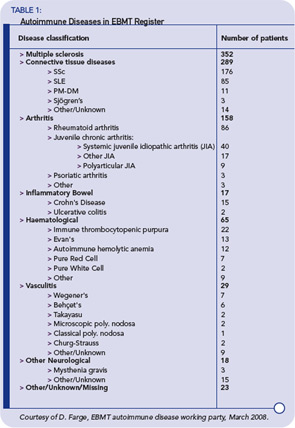
Autologous hematopoetic stem cell transplantation (HSCT) refers to the use of the patient’s own stem cells as a rescue therapy after high-dose myeloablative therapy. In contrast, allogeneic HSCT refers to the use of stem cells from a human leucocyte antigen (HLA)– matched related or unrelated donor. The latter is used for a variety of malignant and nonmalignant disorders to replace a defective host marrow or immune system with the supposedly healthy donor marrow and immune system. More than 10 years ago, the first patients were successfully treated with an autologous HSCT for a life- or organ-threatening autoimmune disease refractory to conventional therapy.1 Table 1 (p. 21) shows the status of the European Group for Blood and Marrow Transplantation (EBMT) Autoimmune Disease Working Party register as of March 2008.
The goal of using autologous HSCT for the treatment of autoimmune diseases is to reduce or eliminate autoreactive cells, mainly of the adaptive immune system, through conditioning with highly active cytotoxic agents.2 Thereafter, newly developing B and T cells may be introduced to autoantigens and undergo self-tolerization; alternatively, the chance environmental circumstances that led to the initial autoimmunity might never happen again in the patient’s lifetime. The concordance rate of autoimmune diseases in identical twins shows that an autoimmune-prone genetic background alone is insufficient for disease expression. These rates are in the range of 15% for systemic lupus erythematosus to 50% for type 1 diabetes mellitus and suggest that this approach could lead to sustained remissions in some patients. Initially, it was thought that complete ablation of autoreacting cells would be required for durable remissions, but more recent immune reconstitution data suggests that a significant “debulking” of autoaggressive immunocytes could allow the normal immune regulation mechanisms to recontrol the system.3
To obtain cells for autologous HSCT before conditioning, stem cells are mobilized from the marrow to the peripheral blood, which permits stem-cell collection without general anesthesia or bone-marrow harvest. This mobilization is achieved by various protocols, many of which involve granulocyte colony-stimulating factor (G-CSF) or cyclophosphamide, a drug that is myelosuppressive and immunosuppressive per se but leads to a “rebound” mobilization of stem cells. The autologous hematopoetic stem cells are then collected through leukapheresis. In a further step, the hematopoetic stem cells expressing CD34+ as a stem-cell marker can be purified through positive selection using CD34 + sorting or negative selection of T or B cells using lineage-specific surface markers. This procedure leads to a more profound T cell depletion of the autograft (up to a CD3+ cell content lower than one T cell per 105 cells in the graft).
Stem-cell mobilization is an immunosuppressive process, especially if cyclophosphamide is used. More profound immunosuppression is subsequently achieved through conditioning before HSCT. Conditioning means that an immunosuppressive regimen causes an at least transient bone marrow insufficiency, leading to a pancytopenia. The aim of conditioning is to eliminate to the greatest extent possible autoreactive cells, mostly T or B cells. The dose-limiting factor for such an intense immunosuppression is the hematotoxicity. However, hematotoxicity is reduced by retransfusion of autologous hematopoetic stem cells after conditioning. In any case, a transient pancytopenia occurs with a nadir around day 10 after HSCT. Transfusion of red cells or platelets may be necessary. Prophylactic antibiotic, antifungal, and antiviral therapy are given depending on the intensity of leuco- or lymphopenia. For conditioning chemotherapy, cyclophosphamide, busulfan or radiotherapy, or antibodies against components of the immune system have been used alone or in combination.
Allogeneic HSCT is associated with GvHD that carries significant morbidity and mortality. Theoretically, allogeneic HSCT offers the replacement of an autoreactive immune system with one that is putatively normal, but, in practice, this has yet to be proven. In fact, there are case reports of allogeneic HSCT being performed in patients with rheumatoid arthritis (RA) suffering from drug-induced aplastic anemia and, despite four years of remission, relapse occurred with all immune competent cells being of donor origin. On the other hand, similar cases have had up to 20 years clinical and serological remission.
Although hematopoetic reconstitution occurs within two weeks after HSCT, complete regeneration of the adaptive immune system, in contrast, is delayed up to years. It is thought that as the immune system returns in the presence of the inciting autoantigen, tolerance occurs.
HSCT is associated with substantial morbidity and mortality. In the beginning, transplant-related mortality for patients with systemic sclerosis (SSc) undergoing HSCT was higher than 10% in retrospective database analyses. In the last years, transplant-related mortality seems to be reduced after patients with advanced organ damage have been excluded from transplantation. Currently, active prospective trials recruit patients with a high risk of an adverse outcome of their autoimmune disease but with a still preserved organ function.
Toxicity of HSCT comes from the induction regimen itself. For example, cyclophosphamide or melphalan are potentially cardiotoxic, especially if used in patients with preexisting heart disease; this can be the case in autoimmune diseases such as SSc. G-CSF used to mobilize stem cells and to shorten engraftment after transplantation has been shown to induce a flare of the autoimmune disease in single cases. Antithymocyte globulin (ATG) used in the conditioning regimen is associated with hypersensitivity reactions.
The desired immunoablative effect of HSCT, on the other hand, comes along with an increased susceptibility to infections. This is the case mostly for bacteria or fungi during aplasia (up to 14 days after induction therapy), and this is also the case during the phase of immune reconstitution that can be delayed up to several months. During the latter, the patients are at risk for opportunistic infections or reactivation of silent viruses.
Results of HSCT for Autoimmune Diseases
Data on the effect of HSCT on patients suffering from autoimmune diseases are obtained from retrospective case series, the EBMT/European League Against Rheumatism (EULAR) database, and from published phase I/II trials.
Up until March 2008, the EBMT/EULAR database included results from nearly 1,000 patients who received an HSCT for the indication autoimmune disease registered. Among these patients, more than 900 received an autologous HSCT and around 50 an allogeneic HSCT. Indication for autologous HSCT have been multiple sclerosis (MS) in 353 patients, SSc in 176, systemic lupus erythematosus (SLE) in 85, rheumatoid arthritis in 86, and other autoimmune diseases in fewer patients. (See Table 1, below left.) Most often, peripheral blood stem cells have been used as a stem-cell source but, in 65 cases, bone marrow was used. In more than one-third of the patients, in vitro CD34 positive selection had been performed.
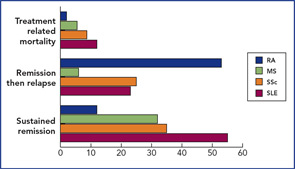
The results of the phase I/II trials and registered case reports have been published.2 In essence, two publications have shown that around 50% of SLE patients achieved remission at some stage post-transplant,4,5 and, in MS and SSc, durable remission occurred in one-third. (See Figure 3, p. 22.) Transplant-related mortality was highest in the multicenter SLE series, but in general, that is now less, due to more stringent patient selection.
In SSc, it has been recently shown that HSCT is not just a once-only immunosuppression. HSCT also seems to be able to reverse fibrosis6,7 and improve micro-vascularisation.8,9
A prospective trial called the Autologous Stem Cell Transplantation International Scleroderma trial (ASTIS) is nearing completion in Europe, comparing HSCT with 12 monthly cyclophosphamide IVI pulses; in the United States, the Scleroderma Cyclophosphamide or Transplant (SCOT) trial is running. Both have similar designs with the exception of the treatment arm of SCOT, which includes limited radiotherapy.
Limited experience is available with allogeneic HSCT for autoimmune disease.10 Although offering the theoretical advantage of a “healthy” immune system, the real risk of GvHD and its attendant morbidity and mortality has limited this form of HSCT to small series and case reports.
The Future of HSCT for Autoimmune Disease
There are now significant data suggesting that a profound immunoreduction supported by autologous stem cell rescue may alter the natural history of some autoimmune disease, with most experience being in SSc. The initial treatment is hazardous, with potentially lethal complications such as cardiotoxicity or overwhelming infection.
So far, however, there is no indication that unknown toxicity occurs in autoimmune disease patients. The considerable toxicity of HSCT in general is the reason that the treatment should only be performed in experienced transplant units. When selecting a patient for such a treatment, one should ask the following questions:
- Is the disease potentially more dangerous than the treatment? (Current estimates are around 5% transplant-related mortality.)
- Is the patient in too advanced a state of vital organ damage such that the chances of meaningful benefit are outweighed by the higher risk of transplant-related mortality (e.g., advanced lung fibrosis with diffusing capacity of the lung for carbon monoxide of less than 30% predicted)?
- Does the patient and his or her family understand that the stem-cell transplant is only to “rescue the normal blood cells” after heavy immunoablation and not to supply some special healing action on the diseased organs?
- Have you exhausted the existing conventional proven treatments for this autoimmune disease?
If the answers to these questions are affirmative, then it is reasonable to enter such a patient into one of the experimental trials. Only through prospective randomized trials are hematologists able to offer long-term cure to certain leukemia patients, even though the exact cause of leukemia remains unknown.
Although anecdotes have a limited place in clinical research, the experience of seeing a young scleroderma patient go from an exhausted and hidebound patient to a near normal individual over three to six months has inspired many of the investigators to push on with the prospective trials to reach the correct conclusion. We owe this to those patients who have put their trust in the program already, not all of whom have done well.
Multipotent MSC for Autoimmune Diseases
Mesenchymal stromal cells (MSCs) are also referred to as mesenchymal stem cells, though their true “stemness” has yet to demonstrated.11 MSCs are capable of differentiating in vitro and in vivo to different MSC lineages, including adipose, bone, cartilage, muscle, and myelosupportive stroma. MSCs may be isolated from bone marrow, skeletal muscle, adipose tissue, synovial membranes, and other connective tissues of human adults, as well as cord blood and placental products, and are defined by using a combination of phenotypic markers and functional properties.
In vitro, MSCs have vast proliferative potential, can clonally regenerate, and can give rise to differentiated progeny. These cells also exhibit anti-proliferative and anti-inflammatory properties in vitro and in vivo, making them candidates for treatment of acute inflammatory autoimmune disease.12 Regardless of whether or not MSCs are true stem cells, clinical benefit from MSCs may not require sustained engraftment of large numbers of cells or differentiation into specific tissues. It is possible that a therapeutic benefit can be obtained by local paracrine production of growth factors and a provision of temporary antiproliferative and immunomodulatory properties.
MSCs enjoy a degree of immune privilege in that allogeneic MSCs may be infused into patients without any preconditioning and seem to survive long enough to exert positive clinical effects without acute toxicity.
The mechanisms underlying the immunosuppressive effect of MSCs remain to be fully clarified, with sometimes-conflicting data probably reflecting the variable definitions and experimental conditions. Certainly, mechanisms may be multiple and involve both cell contact and the production of soluble factors including interleukin (IL)-10, transforming growth factor-beta, hepatocyte growth factor, IL-1 receptor antagonist, and soluble HLA-G.
Animal Models
It may be impossible to try to separate the anti-inflammatory, immunomodulatory, and tissue protective “trophic” effects of MSC. An immunosuppressive effect of MSC in vivo was first suggested in a baboon model, where infusion of ex vivo expanded donor or third-party MSCs delayed the time to rejection of histoincompatible skin grafts. In murine models, MSCs can also down-regulate bleomycin-induced lung inflammation and fibrosis, if given early (but not late) after the induction. A similar effect was observed in a murine hepatic fibrosis model (carbon tetrachloride-induced) using an MSC line. Tissue protective effects were also seen in a rat kidney model of ischemia/reperfusion injury in which syngeneic MSCs were used.
In the two murine models of experimental autoimmune encephalomyelitis, both clinical and histological improvement occurred. However, in a murine model of arthritis, collagen induced arthritis was not improved by the addition of MSCs and the in vitro immunosuppressive effects were reversed by the addition of tumor necrosis factor-α. MSCs were not found in the joints. A second murine arthritis model showed, however, a positive outcome.
Recently, a murine model of streptozocin-induced diabetes mellitus was reported to improve clinically following transplantation with a combination of bone marrow derived cells and syngeneic and allogeneic MSCs following sublethal irradiation. The proposed mechanism was regeneration of recipient derived islet cells plus immunosuppression of autoreactive T cells. Neither cell product alone was effective.
MSCs and Human Experience
Ex vivo–expanded allogeneic MSCs have been infused in several phase I studies. No adverse events during or after MSC infusion have been observed, and no ectopic tissue formation has been noted. After infusion, MSCs remain in the circulation for no more than an hour. In another study, infusion of haploidentical MSCs to a patient with steroid-resistant severe acute GvHD of the gut and liver promptly improved liver values and intestinal function.
The EBMT is currently running protocols for prevention and treatment of acute GvHD through its developmental committee, and interim results of the treatment trial have recently been published. Thirty out of 55 steroid-resistant acute-GvHD patients had a complete response with no immediate toxicity.13
Autologous bone marrow derived MSCs have been shown to be potently antiproliferative to stimulated T cells from normal subjects and autoimmune (e.g., RA, SSc, Sjögren’s, SLE) patients; in SSc patients, these MSCs were normal in respect to proliferation, clonogenicity, and differentiation to bone and fat.14,15
Currently, few peer-reviewed publications concerning the results of using of MSCs in human autoimmune disease are available. There are several ongoing phase I/II clinical trials in autoimmune disease including MS, with discussions underway concerning other trials for other autoimmune disease such as type 1 diabetes mellitus, SSc, vasculitis, and SLE.
Important is the setting of clear therapeutic targets and harmonization of cell products, especially MSC source and type (autologous or allogeneic), cell expansion conditions, and trial protocols. In addition, long-term safety data collection across disciplines is required, and an international interdisciplinary registry of MSC-treated patients has been launched.
Summary
Cellular therapy using hematopoietic stem cells to support the ablated normal blood cells has enabled crossing a threshold of immunoablation, and early results suggest that a “resetting” of the immune system in patients with autoimmune disease beyond just immunosuppression is possible. Only phase III randomized trials will establish the true value of this approach. In contrast to the goals of treatment with HSC, MSCs are being used as “homing and healing” cells in various severe inflammatory settings including autoimmune disease, with phase I/II studies beginning. Importantly, no patient conditioning is required. Certainly, for diseases such as SSc where existing immunosuppressive has not been highly effective, stem-cell therapy may offer a fundamentally new approach to correct immune abnormalities and initiate repair. Although much work on stem cells needs to be performed in both the clinical and laboratory, there is strong optimism that a potential new era of therapy is now at hand.
Dr. Tyndall is professor and head of the department of rheumatology at the University of Basel, Felix Platter Hospital, in Basel, Switzerland.
References
- 1. Tamm M, Gratwohl A, Tichelli A, Perruchoud AP, Tyndall A. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Ann Rheum Dis. 1996; 55:779–780.
- 2. van Laar JM, Tyndall A. Adult stem cells in the treatment of autoimmune diseases. Rheumatology (Oxford). 2006; 45:1187–1193.
- 3. Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005; 201:805–816.
- 4. Jayne D, Passweg J, Marmont A, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004; 13:168–176.
- 5. Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006; 295:527–535.
- 6. Verrecchia F, Laboureau J, Verola O, et al. Skin involvement in scleroderma—where histological and clinical scores meet. Rheumatology (Oxford). 2007; 46:833–841.
- 7. Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: Long-term follow-up of the US multicenter pilot study. Blood. 2007; 110:1388–1396.
- 8. Fleming JN, Nash RA, McLeod DO, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE. 2008;3(1):e1452.
- 9. Aschwanden M, Daikeler T, Jaeger KA, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008; 67:1057–1059.
- 10. Griffith LM, Pavletic SZ, Tyndall A, et al. Target populations in allogeneic hematopoietic cell transplantation for autoimmune diseases—a workshop accompanying: Cellular therapy for treatment of autoimmune diseases, basic science and clinical studies, including new developments in hematopoietic and mesenchymal stem cell therapy. Biol Blood Marrow Transplant. 2006; 12:688–690.
- 11. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004; 32:414–425.
- 12. Dazzi F, van Laar JM, Cope A, Tyndall A. Cell therapy for autoimmune diseases. Arthritis Res Ther. 2007;9:206.
- 13. Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008; 371:1579–1586.
- 14. Bocelli-Tyndall C, Bracci L, Spagnoli G, et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford). 2007; 46:403–408.
- 15. Larghero J, Farge D, Braccini A, et al. Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann Rheum Dis. 2008; 67:443–449.