NEW YORK (Reuters Health)—After an immune-related adverse event, the risk-reward ratio for an anti-PD-1 (anti-programmed death-1) or anti-PD-L1 (anti-programmed death ligand-1) rechallenge seems to be acceptable if patients are closely monitored, researchers say.
“The immune checkpoint inhibitors anti-PD-1 and anti-PD-L1 have proven efficacy in the treatment of many cancers, but patients may experience immune-related adverse events (irAEs). (Treatment) is usually stopped when a grade 2 or higher irAE occurs,” Dr. Olivier Lambotte of Hopitaux Universitaires Paris Sud AP-HP in France told Reuters Health.
“We were able to study 93 patients with a broad spectrum of cancers in whom a rechallenge was [considered],” he said by email. “Among them, 40 patients [43%] were rechallenged with the same anti-PD-1 or anti-PD-L1.”
“Earlier initial toxicity was associated with more frequent irAE recurrence,” he noted. However, “the second irAEs were not more severe than the first. Thus, the risk-reward ratio for an anti-PD-1 rechallenge appears to be acceptable, but each individual situation has to be taken into account.”
As reported online June 6 in JAMA Oncology, patients’ median age was 62.5, about half were women, and the main cancer types/tumor sites were melanoma (33%), lung (16%), colorectal (9%), and lymphoma (9%). The main outcome was incidence of a second irAE after readministration of an anti-PD-1 or anti-PD-L1 after an initial grade 2 or higher irAE.
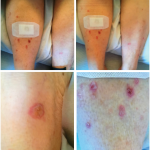
Initial irAEs consisted of 43 grade 2 events (46%), 36 grade 3 events (39%), and 14 grade 4 events (15%), presenting primarily as hepatitis (18%), skin toxicity (15%), pneumonitis (14%), colitis (12%), and arthralgia (7.5%).
As Dr. Lambotte noted, 43% of patients were rechallenged with the same anti-PD-1 or anti-PD-L1 agent. The rechallenged and non-rechallenged groups did not differ in terms of median age (61 vs. 63, respectively), time to initial irAE (5 vs. 3 treatment cycles), irAE severity (about half in each group experienced grade 2 and half, grades 3-4), or steroid use (42.5% vs. 60%).
During a median follow-up of 14 months, the same irAE or a different irAE occurred in 22 patients (55%). Shorter time to the initial irAE (9 vs. 15 weeks) was associated with the occurrence of a second irAE, but this was not more severe than the first.
“Rechallenge is a good example of the importance of setting up multidisciplinary boards to assess the management of severe irAEs and to discuss whether or not to rechallenge,” Dr. Lambotte said. “In some patients, the rechallenge is the last possible therapeutic option. In these patients, the rechallenge is important to discuss. On the other hand, in an adjuvant situation, the risk-reward ratio is not for the rechallenge.”
Dr. Igor Puzanov, Chief of Melanoma and Director of the Early Phase Clinical Trials Program at Roswell Park Comprehensive Cancer Center in Buffalo, New York, commented by email, “This is an important study, and includes useful safety information for practicing oncologists. The findings let us know that toxicity itself is not a deal-breaker in terms of continuing a patient on a checkpoint inhibitor after an immune-related adverse event.”
“I agree fully with the strategy of intensive monitoring of this group of patients,” he told Reuters Health. “At Roswell Park, we have instituted formal monitoring for cardiac toxicity, doing weekly troponin checks for six to 12 weeks in all patients starting on a checkpoint inhibitor.”
“I would add that if a patient experiences toxicity from anti-PD-1 therapy, you manage the toxicity first, and if you need to you can consider restarting the anti-PD-1 later,” he said. “If the patient had a high-grade toxicity, you would still manage the toxicity first but should wait to see if you even need to restart the anti-PD-1 treatment, because we’ve seen that a lot of patients do well with just observation but without further treatment on a checkpoint inhibitor. If the tumor is stable, you can just watch and wait, and if they have some progression in the future then you can consider restarting the checkpoint inhibitor later.”