Nongenomic cytosolic receptor-mediated mechanism: Rapid, nongenomic effects of glucocorticoids can be mediated by dissociation of the existing cGCR–multiprotein complex caused by binding of glucocorticoids to the cGCR. Released proteins, such as Src, are thought to be responsible for some of the rapid effects of glucocorticoids. For example, studies have shown that the rapid effect of glucocorticoids that are mediated by cGCR occupation (other than those mediated by changes in gene transcription) lead to a failure of recruitment of signalling factors and therefore to impaired signalling cascades.4
Nongenomic membrane-bound receptor-mediated mechanism: Another possible mechanism for mediating nongenomic glucocorticoid effects is via the mGCR. These receptors have been detected on human peripheral blood mononuclear cells in small numbers using highly sensitive immunofluorescent staining with the same monoclonal antibody as for the cGCR. In patients with RA, the number of mGCR-positive monocytes correlates with disease activity. The mGCR has also been shown to be upregulated in monocytes and B lymphocytes of patients with ankylosing spondylitis. However, this upregulation did not correlate with the humoral or overall disease activity. In a study of patients with systemic lupus erythematosus, the frequencies of mGCR+ monocytes (CD14+) were considerably higher than those in healthy controls and were inversely correlated with glucocorticoid dosages. Together, these data suggest that although mGCRs may be involved in the pathophysiology of rheumatic diseases and perhaps also other inflammatory conditions, the origin and function of the receptor are currently still unknown.
Nongenomic nonspecific mechanism: At high concentrations, glucocorticoids can alter the physicochemical properties of biological membranes, especially plasma and mitochondrial membranes, via a nongenomic mechanism. Glucocorticoids are thought to intercalate into these membranes and to change the function of membrane-associated proteins, thereby affecting lipid peroxidation with or without affecting membrane permeability. In immune cells, membrane association of glucocorticoids results in rapidly reduced calcium and sodium cycling across the plasma membranes, which, in turn, may contribute to immunosuppression and the reduction of inflammation.
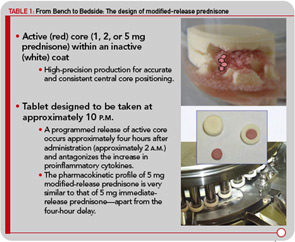
Improving the Risk–Benefit Ratio for Treatment with Glucocorticoids
Dr. Hench first used physiologic, but pharmaceutically manufactured, cortisone successfully in a patient with RA in 1948. However, he soon became aware of its mineralocorticoid adverse effects—sodium/water retention and potassium loss.5 Since then, the exact role and benefits of glucocorticoids in the treatment of RA have been under considerable debate. However, it is clear that glucocorticoids can produce numerous side effects (e.g., osteoporosis, myopathy, skin atrophy, edema, glaucoma), especially if given at high dosages for longer periods.6 As a consequence, a first approach to address this limitation was the synthesis of new therapeutic glucocorticoid drugs in the 1950s and 1960s. These drugs differed from Dr. Hench’s original cortisone by having less mineralocorticoid activity but also by exhibiting significantly more glucocorticoid potency. Examples of these drugs are well known and still widely used, such as prednisone/prednisolone (1955) and methylprednisolone (1957); other drugs of this type include the fluorinated glucocorticoids such as dexamethasone and betamethasone. These synthetic drugs differ from endogenous glucocorticoids in terms of plasma kinetics, metabolism, biological half-life, lipophilicity, drug-receptor interactions, and nongenomic potencies.5
A second sensible approach to improve treatments with glucocorticoids has been well established, reflecting the realization glucocorticoids could be delivered directly to the site of inflammation (e.g., by way of intraarticular injections). For the various approaches to glucocorticoid therapy, recommendations and guidelines to optimize dosing have been developed with all guidance offering the obvious advice to give as much glucocorticoid as necessary but as little as possible. More recently, novel glucocorticoids or glucocorticoid receptor ligands with highly targeted actions have been synthesized. Selective glucocorticoid receptor agonists (SEGRAs) and nitrosteroids represent examples of this approach.7