In 1950, Philip S. Hench and his co-workers Edward Kendall and Tadeus Reichstein received the Nobel Prize for medicine for their landmark discovery of glucocorticoids and the beneficial effects in rheumatoid arthritis (RA). Since that time, glucocorticoids have become one of the most commonly prescribed classes of drugs in all of medicine, although many issues about optimal use remain surprisingly unknown. This article summarizes the current knowledge on the mode of action of glucocorticoids and highlights approaches to improve the risk–benefit ratio of these important drugs, as well as the development of novel glucocorticoids or glucocorticoid receptor ligands. In this regard, the focus of this discussion will be on a new concept in drug administration termed chronotherapy with modified-release (MR) prednisone for the treatment of RA.
Glucocorticoids’ Mode of Action
As shown in extensive studies in vivo and in vitro, glucocorticoids mediate their antiinflammatory and immunosuppressive effects by four different mechanisms that, unfortunately, also produce their serious adverse effects. These mechanisms include:
- The classical genomic mechanism resulting from activation of the cytosolic glucocorticoid receptor (cGCR);
- Secondary nongenomic effects initiated by the cGCR;
- Membrane-bound glucocorticoid receptor (mGCR)– mediated nongenomic effects; and
- Nonspecific, nongenomic effects caused by interactions with cellular membranes.1-3

Each of these mechanisms will be considered in turn.
Glucocorticoid Mechanisms of Action
Classical genomic mechanism: The term classical genomic refers to the most important mechanism of glucocorticoid action that results in changes in gene expression via transactivation and transrepression. This mechanism involves passage of glucocorticoid molecules through the plasma membrane, high-affinity binding of these molecules to the inactive cGCR, formation of the activated glucocorticoid/cGCR complex, and translocation of the complex into the nucleus. The term transactivation refers to transcriptional transactivation by binding of a dimerized glucocorticoid receptor (GR) protein complex to the promoter of glucocorticoid-regulated genes, ultimately leading to upregulated synthesis of certain regulatory proteins. The interference with the activity of (proinflammatory) transcription factors (such AP1, NF-қB, or IRF-3) by GR monomers is called transrepression and leads to the down-regulation of (proinflammatory) protein synthesis. It should be stressed that these processes require time to occur—30 minutes or more pass before significant changes are recognized in regulator protein concentrations. It usually takes hours or days before changes on a cell, tissue, or organ level become evident. In contrast, nongenomic effects occur rapidly, within seconds or minutes.
Nongenomic cytosolic receptor-mediated mechanism: Rapid, nongenomic effects of glucocorticoids can be mediated by dissociation of the existing cGCR–multiprotein complex caused by binding of glucocorticoids to the cGCR. Released proteins, such as Src, are thought to be responsible for some of the rapid effects of glucocorticoids. For example, studies have shown that the rapid effect of glucocorticoids that are mediated by cGCR occupation (other than those mediated by changes in gene transcription) lead to a failure of recruitment of signalling factors and therefore to impaired signalling cascades.4
Nongenomic membrane-bound receptor-mediated mechanism: Another possible mechanism for mediating nongenomic glucocorticoid effects is via the mGCR. These receptors have been detected on human peripheral blood mononuclear cells in small numbers using highly sensitive immunofluorescent staining with the same monoclonal antibody as for the cGCR. In patients with RA, the number of mGCR-positive monocytes correlates with disease activity. The mGCR has also been shown to be upregulated in monocytes and B lymphocytes of patients with ankylosing spondylitis. However, this upregulation did not correlate with the humoral or overall disease activity. In a study of patients with systemic lupus erythematosus, the frequencies of mGCR+ monocytes (CD14+) were considerably higher than those in healthy controls and were inversely correlated with glucocorticoid dosages. Together, these data suggest that although mGCRs may be involved in the pathophysiology of rheumatic diseases and perhaps also other inflammatory conditions, the origin and function of the receptor are currently still unknown.
Nongenomic nonspecific mechanism: At high concentrations, glucocorticoids can alter the physicochemical properties of biological membranes, especially plasma and mitochondrial membranes, via a nongenomic mechanism. Glucocorticoids are thought to intercalate into these membranes and to change the function of membrane-associated proteins, thereby affecting lipid peroxidation with or without affecting membrane permeability. In immune cells, membrane association of glucocorticoids results in rapidly reduced calcium and sodium cycling across the plasma membranes, which, in turn, may contribute to immunosuppression and the reduction of inflammation.
Improving the Risk–Benefit Ratio for Treatment with Glucocorticoids
Dr. Hench first used physiologic, but pharmaceutically manufactured, cortisone successfully in a patient with RA in 1948. However, he soon became aware of its mineralocorticoid adverse effects—sodium/water retention and potassium loss.5 Since then, the exact role and benefits of glucocorticoids in the treatment of RA have been under considerable debate. However, it is clear that glucocorticoids can produce numerous side effects (e.g., osteoporosis, myopathy, skin atrophy, edema, glaucoma), especially if given at high dosages for longer periods.6 As a consequence, a first approach to address this limitation was the synthesis of new therapeutic glucocorticoid drugs in the 1950s and 1960s. These drugs differed from Dr. Hench’s original cortisone by having less mineralocorticoid activity but also by exhibiting significantly more glucocorticoid potency. Examples of these drugs are well known and still widely used, such as prednisone/prednisolone (1955) and methylprednisolone (1957); other drugs of this type include the fluorinated glucocorticoids such as dexamethasone and betamethasone. These synthetic drugs differ from endogenous glucocorticoids in terms of plasma kinetics, metabolism, biological half-life, lipophilicity, drug-receptor interactions, and nongenomic potencies.5
A second sensible approach to improve treatments with glucocorticoids has been well established, reflecting the realization glucocorticoids could be delivered directly to the site of inflammation (e.g., by way of intraarticular injections). For the various approaches to glucocorticoid therapy, recommendations and guidelines to optimize dosing have been developed with all guidance offering the obvious advice to give as much glucocorticoid as necessary but as little as possible. More recently, novel glucocorticoids or glucocorticoid receptor ligands with highly targeted actions have been synthesized. Selective glucocorticoid receptor agonists (SEGRAs) and nitrosteroids represent examples of this approach.7
A third successful and recent approach to improve the risk–benefit ratio is to refine treatment with conventional glucocorticoids. One option is to combine a very low dose of prednisolone with dipyridamole, which can enhance antiinflammatory activities in immune cells.8 Another interesting approach is the targeted delivery of glucocorticoids to sites of inflammation using liposomes such as a polyethylene glycol (PEG) carrier system. In this delivery technique, the glucocorticoid is enclosed in small vesicles (~100 nm in size) that accumulate at the site of inflammation. As a consequence, very high local concentrations are achieved for increased periods of time. Animal studies have demonstrated that, in terms of clinical effectiveness, liposomal glucocorticoids are superior to conventional high-dose glucocorticoid therapy delivered intravenously.9,10 Because of the encapsulation of the drug and correspondingly lower plasma levels, liposomal administration is expected to have lower adverse effects than that of conventional glucocorticoid therapy. Recently, it has been reported that the use of larger liposomes (~295 nm in size) using non-PEGylated liposomal dexamethasone phosphate is effective and allows persistent therapeutic effects with separation of risks and benefits in animal arthritis models.11,12
The fourth and by far the most advanced approach to optimize treatment with current glucocorticoids involves the programmed delivery of glucocorticoids using MR prednisone. This specific formulation changes the timing of active drug release. The clinical relevance of the timing of glucocorticoid administration, (i.e., of chronotherapy with MR prednisone) is assessed here.
Chronotherapy with MR Prednisone
A circadian rhythm of pain, stiffness, and functional disability, as well as the underlying cyclic variations in hormone levels and cytokine concentrations, are well-known phenomena in patients with RA.13 Similar diurnal variations have been described for other rheumatic diseases such as polymyalgia rheumatica and ankylosing spondylitis.14 Although data for the latter conditions are limited, the mechanisms underlying the development of morning symptoms in RA are increasingly well understood. In this disease, major symptoms such as pain, inflammation, and stiffness vary as a function of the time of day, usually with the highest severity in the morning hours. These symptoms are preceded by elevated levels of interleukin-6 (IL-6) and other proinflammatory cytokines.13,15–18 A causal and not only a temporal relationship between these cytokines and symptoms has been suggested since the pathogenesis of RA involves complex humoral and cellular reactions including immune complex formation, vascular reactions, and infiltration of lymphocytes and monocytes into the synovium.19
Online Resource
The ACR recently published “2010 Recommendations for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis” (Arthritis Care Res. 2010;62:1515-1526), which are available at www.rheumatology.org in the Practice Management menu.
Based upon these considerations, it has been suggested that varying the timing of glucocorticoid administration to coincide better with circadian rhythms could help improve therapy for RA.20 The scientific basis for this hypothesis is provided by the following three key points:
- Pain, fatigue, morning stiffness, and immobility are common symptoms affecting patient quality of life and the ability to stay gainfully employed.21
- The overnight rise in IL-6 and other proinflammatory cytokines is thought to initiate a cascade of events resulting in these symptoms.
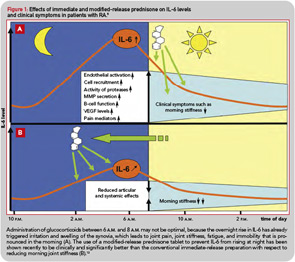
- Preventing the nocturnal rise of IL-6 and other proinflammatory cytokines should be more effective than treating established symptoms. From this point of view, the conventional administration of glucocorticoids between 6 a.m. and 8 a.m. may not be optimal, because it is too late to target the effects of nocturnal proinflammatory stimuli (see Figure 1A).
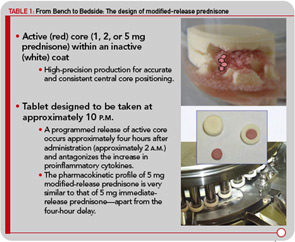
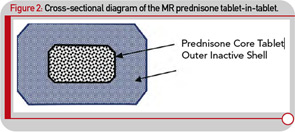
Although administering a standard glucocorticoid drug prior to the rise of cytokine synthesis and inflammatory activity could theoretically enhance efficacy, in practical terms, this approach would necessitate having the patient wake up during the night to take the drug, because conventional glucocorticoids have only a short half-life. Evidence that timing of exogenous glucocorticoid administration can improve treatment benefits was provided by Arvidson and co-workers in a study in the late 1990s. This study showed that low doses of prednisolone taken at 2 a.m. had more effect on morning symptoms of RA than achieved by the equivalent dose taken at 7:30 a.m. (see Figure 1B).17 However, having patients awake each night at about 2 a.m. is clearly not a feasible long-term treatment option. Therefore, a MR prednisone tablet was developed to enable prednisone chronotherapy for RA, in which the delivery of treatment is coordinated with biological rhythms (see Figure 1B). This new tablet releases prednisone approximately four hours after ingestion, (i.e., at approximately 2 a.m. if taken at bedtime) as indicated in Table 1. The MR prednisone is in a tablet-in-tablet dosage form, consisting of an immediate-release prednisone core tablet surrounded by an inactive outer tablet shell (see Figure 2). Prednisone release is triggered by penetration of gastrointestinal fluid into the tablet shell and is independent of the gastrointestinal milieu (like pH). The tablet strengths of 1, 2, and 5 mg are distinguished from one another by both color and debossing.
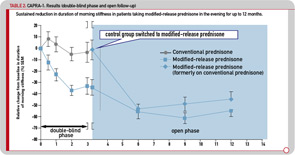
The efficacy and safety of this novel medication was investigated in a three-month, double-blind, double-dummy, randomized controlled clinical study (Circadian Administration of Prednisone in Rheumatoid Arthritis, also known as the CAPRA-1 study). In total, 288 patients who had active RA and were already receiving chronic prednisone therapy were randomized to receive their current dose (2.5–10 mg prednisone per day) either as MR prednisone administered at approximately 10 p.m. or as conventional immediate-release (IR) prednisone administered in the morning. All patients continued on their background therapy with disease-modifying antirheumatic drugs (DMARDs) and nonsteroidal antiinflammatory drugs (NSAIDs).13 In this study, the new formulation was shown to be clinically superior to the conventional IR preparation with respect to reducing morning joint stiffness, which was the primary endpoint of this study (see Figure 1B). IL-6 serum concentrations also were significantly decreased by MR prednisone after three months of treatment but remained unchanged by IR prednisone. The safety profile did not show differences between the two preparations.13
The initial three-month, double-blind phase was followed by a nine-month, open-label extension period, during which all patients were treated with MR prednisone. The reduction in morning stiffness duration and IL-6 serum levels observed during the double-blind phase in patients treated with MR prednisone was sustained during 12 months of treatment, with the duration of morning stiffness reduced from baseline by approximately 50% (see Table 2). Similar improvements were observed in patients who switched to MR prednisone in the open-label phase, and 37% of all patients achieved improvement according to the ACR20 criteria.22 It was concluded that MR prednisone is well tolerated and convenient to administer. The results of the 12-month CAPRA-1 study showed that long-term, low-dose MR prednisone chronotherapy results in a significant reduction in morning joint stiffness in addition to all known therapeutic effects with conventional prednisone.
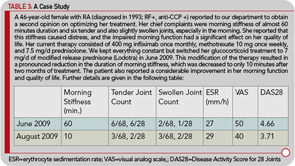
The hypothalamic–pituitary–adrenal (HPA) axis plays an important role in regulating and controlling immune responses, and dysfunction of the axis has been implicated in the pathogenesis of RA and other rheumatic diseases. The influence of prednisone therapy on the HPA axis function is still a matter of concern, particularly for longer treatment durations, although evidence on the effects is rather limited. Hence, as part of the CAPRA-1 study, to investigate the influence of long-term, low-dose prednisone chronotherapy with MR prednisone on the HPA axis, corticotrophin releasing– hormone (CRH) tests were performed in a subgroup of 28 patients. The CRH tests were performed at three time points during the study (baseline, at the end of the double-blind phase, and at the end of the nine-month, open-label extension). There was no indication that changing treatments from IR prednisone to chronotherapy with MR prednisone increased the risk of HPA axis insufficiency, or deterioration of preexisting suppression. In addition, no adverse events that could be attributed to HPA axis insufficiency were observed during the treatment with low-dose MR prednisone for the entire treatment period of 12 months.23
A second, large-scale, multicenter study was performed (CAPRA-2) as a rigorous placebo-controlled study to investigate the efficacy of low-dose MR prednisone. In this 12-week, double-blind, placebo-controlled study, 350 RA patients were randomized to 5 mg MR prednisone (n=231) or placebo (n=119) once daily in addition to their standard DMARD treatment. As such, the net effect of low-dose prednisone chronotherapy in patients with RA poorly controlled by DMARDs was quantified. The initial data were presented at the European League Against Rheumatism meeting 2010 in Rome.24,25 At 12 weeks, profound improvements in symptoms and function were achieved with low-dose prednisone chronotherapy compared with placebo. The ACR20 response rate at 12 weeks was 48% for MR prednisone versus 29% for placebo (P<0.001), and significant improvements were evident within two weeks of therapy initiation. Importantly, improvements in health-related quality of life, in particular physical functioning and fatigue, were also noted, while the safety profile of low-dose prednisone chronotherapy was comparable to that of placebo (data submitted for publication).
Conclusions
Glucocorticoids are highly effective antiinflammatory and immunosuppressive drugs that are frequently used in clinical medicine, often with significant benefits. The potential of these agents to produce adverse effects, however, is notable and has become a driving force for interesting and novel attempts to improve the risk–benefit ratio. The most advanced of these approaches is chronotherapy with prednisone; in chronotherapy, the programmed delivery of MR prednisone is coordinated with biological rhythms. The effects of optimizing the timing of glucocorticoid administration with this new formulation have been studied in two large-scale trials (CAPRA-1 and 2), and the data obtained support the view that MR prednisone improves the risk–benefit ratio of long-term, low-dose glucocorticoid treatment in patients with RA.
Although mainstays in the treatment of RA for over 60 years, glucocorticoids remain both friends and enemies and are surprisingly poorly understood. Can chronotherapy make these venerable agents more friendly? The answer to this question is obvious: Only time will tell.
Dr. Buttgereit is professor of rheumatology and clinical immunology at Charité University Medicine Berlin, Germany.
References
- Buttgereit F, Wehling M, Burmester G. A new hypothesis of modular glucocorticoid actions: Steroid treatment of rheumatic diseases revisited. Arthritis Rheum. 1998;41: 761-767.
- Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: An update on the mechanisms of action. Arthritis Rheum. 2004;50:3408-3417.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723.
- Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289-298.
- Buttgereit F, Burmester GR, Straub RH, Seibel MJ, Zhou H. Exogenous and endogenous glucocorticoids in rheumatic diseases. Arthritis Rheum. 2010 Oct 8 (Epub ahead of print).
- Da Silva JA, Jacobs JW, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: Published evidence and prospective trial data. Ann Rheum Dis. 2006;65:285-293.
- Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525-533.
- Zimmermann GR, Avery W, Finelli AL, Farwell M, Fraser CC, Borisy AA. Selective amplification of glucocorticoid anti-inflammatory activity through synergistic multi-target action of a combination drug. Arthritis Res Ther. 2009;11:R12.
- Barrera P, et al. Long-circulating liposomal prednisolone versus pulse intramuscular methylprednisolone in patients with active rheumatoid arthritis [abstract]. Proceedings of the American College of Rheumatology (ACR) Scientific Meeting; Oct 24–29, 2008; San Francisco. Hall A, poster 453, 2008.
- Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059-2066.
- Rauchhaus U, Kinne RW, Pohlers D, et al. Targeted delivery of liposomal dexamethasone phosphate to the spleen provides a persistent therapeutic effect in rat antigen-induced arthritis. Ann Rheum Dis. 2009;68:1933-1934.
- Rauchhaus U, Schwaiger FW, Panzner S. Separating therapeutic efficacy from glucocorticoid side-effects in rodent arthritis using novel, liposomal delivery of dexamethasone phosphate: Long-term suppression of arthritis facilitates interval treatment. Arthritis Res Ther. 2009;11:R190.
- Buttgereit F, Doering G, Schaeffler A, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): A double-blind, randomised controlled trial. Lancet. 2008;371:205-214.
- Spies CM, Cutolo M, Straub RH, Burmester GR, Buttgereit F. More night than day—circadian rhythms in polymyalgia rheumatica and ankylosing spondylitis. J Rheumatol. 2010;37: 894-899.
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007;56:399-408.
- Cutolo, M, Masi AT. Circadian rhythms and arthritis. Rheum Dis Clin North Am. 2005; 31: 115-129, ix-x.
- target=”_blank”>Arvidson NG, Gudbjörnsson B, Elfman L, Rydén AC, Tötterman TH, Hällgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis. 1994;53:521-524.
- Perry MG, Kirwan JR, Jessop DS, Hunt LP. Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Ann Rheum Dis. 2009;68:63-68.
- Dayer JM, Choy E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology (Oxford). 2010;49:15-24.
- Hoes JN, Jacobs JW, Boers M, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007;66:1560-1567.
- Westhoff G, Buttgereit F, Gromnica-Ihle E, Zink A. Morning stiffness and its influence on early retirement in patients with recent onset rheumatoid arthritis. Rheumatology (Oxford). 2008;47: 980-984.
- Buttgereit F, Doering G, Schaeffler A, et al. Targeting pathophysiological rhythms: Prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1275-1280.
- Alten R, Döring G, Cutolo M, et al. Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. J Rheumatol. 2010; 37:2025-2031.
- Buttgereit F, et al. Low-dose glucocorticoid chronotherapy of rheumatoid arthritis: 12 week efficacy data of 5 mg modified-release (MR) prednisone. Ann Rheum Dis. 2010; 69(Suppl 3):220.
- Buttgereit F, et al. Safety data for low-dose glucocorticoid chronotherapy of rheumatoid arthritis with 5 mg modified-release (MR) prednisone in a randomized, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010; 69 (Suppl 3):219.