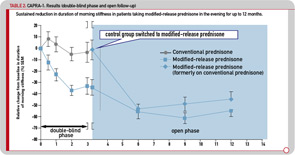
The initial three-month, double-blind phase was followed by a nine-month, open-label extension period, during which all patients were treated with MR prednisone. The reduction in morning stiffness duration and IL-6 serum levels observed during the double-blind phase in patients treated with MR prednisone was sustained during 12 months of treatment, with the duration of morning stiffness reduced from baseline by approximately 50% (see Table 2). Similar improvements were observed in patients who switched to MR prednisone in the open-label phase, and 37% of all patients achieved improvement according to the ACR20 criteria.22 It was concluded that MR prednisone is well tolerated and convenient to administer. The results of the 12-month CAPRA-1 study showed that long-term, low-dose MR prednisone chronotherapy results in a significant reduction in morning joint stiffness in addition to all known therapeutic effects with conventional prednisone.
The hypothalamic–pituitary–adrenal (HPA) axis plays an important role in regulating and controlling immune responses, and dysfunction of the axis has been implicated in the pathogenesis of RA and other rheumatic diseases. The influence of prednisone therapy on the HPA axis function is still a matter of concern, particularly for longer treatment durations, although evidence on the effects is rather limited. Hence, as part of the CAPRA-1 study, to investigate the influence of long-term, low-dose prednisone chronotherapy with MR prednisone on the HPA axis, corticotrophin releasing– hormone (CRH) tests were performed in a subgroup of 28 patients. The CRH tests were performed at three time points during the study (baseline, at the end of the double-blind phase, and at the end of the nine-month, open-label extension). There was no indication that changing treatments from IR prednisone to chronotherapy with MR prednisone increased the risk of HPA axis insufficiency, or deterioration of preexisting suppression. In addition, no adverse events that could be attributed to HPA axis insufficiency were observed during the treatment with low-dose MR prednisone for the entire treatment period of 12 months.23
A second, large-scale, multicenter study was performed (CAPRA-2) as a rigorous placebo-controlled study to investigate the efficacy of low-dose MR prednisone. In this 12-week, double-blind, placebo-controlled study, 350 RA patients were randomized to 5 mg MR prednisone (n=231) or placebo (n=119) once daily in addition to their standard DMARD treatment. As such, the net effect of low-dose prednisone chronotherapy in patients with RA poorly controlled by DMARDs was quantified. The initial data were presented at the European League Against Rheumatism meeting 2010 in Rome.24,25 At 12 weeks, profound improvements in symptoms and function were achieved with low-dose prednisone chronotherapy compared with placebo. The ACR20 response rate at 12 weeks was 48% for MR prednisone versus 29% for placebo (P<0.001), and significant improvements were evident within two weeks of therapy initiation. Importantly, improvements in health-related quality of life, in particular physical functioning and fatigue, were also noted, while the safety profile of low-dose prednisone chronotherapy was comparable to that of placebo (data submitted for publication).
Conclusions
Glucocorticoids are highly effective antiinflammatory and immunosuppressive drugs that are frequently used in clinical medicine, often with significant benefits. The potential of these agents to produce adverse effects, however, is notable and has become a driving force for interesting and novel attempts to improve the risk–benefit ratio. The most advanced of these approaches is chronotherapy with prednisone; in chronotherapy, the programmed delivery of MR prednisone is coordinated with biological rhythms. The effects of optimizing the timing of glucocorticoid administration with this new formulation have been studied in two large-scale trials (CAPRA-1 and 2), and the data obtained support the view that MR prednisone improves the risk–benefit ratio of long-term, low-dose glucocorticoid treatment in patients with RA.