Andrew Brookes / Image Source on Offset
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options; others have few or only off-label options. This series, Rheumatology Drugs at a Glance, provides streamlined information on the administration of biologic, biosimilar and other medications used to treat patients with rheumatic disease.
In Part 5 of this series, we discuss the management of axial spondyloarthritis (axSpA), compile insights from three expert clinicians who have achieved distinction in the field of rheumatology and discuss the 16 available options to treat axSpA (see Table 1). The updated ACR axSpA guideline was published in 2019.1
WHAT THE EXPERTS SAY
Lianne S. Gensler, MD, is a professor of medicine at University of California, San Francisco (UCSF), the director of the spondyloarthritis research program and clinic and the program director for the Rheumatology Fellowship Program. Dr. Gensler served as chair of the Spondyloarthritis Research and Treatment Network (SPARTAN) from 2016 to 2018 and currently serves on the executive committee for the Assessment of SpondyloArthritis International Society (ASAS). She has been the recipient of the Ira M. Goldstein Teaching Award in Rheumatology, the Spondylitis Association Young Investigator Award and the UCSF Research Mentoring Award. She has also been inducted into the Council of Master Clinicians at UCSF.
Mark Hwang, MD, is an assistant professor of medicine at the John P. and Kathrine G. McGovern Medical School at the University of Texas Health Science Center at Houston (UTHSC-H). He is a KL2 Scholar in the UTHSC-H Center for Clinical and Translational Sciences KL2 program. He co-directs the UTHSC-H Spondyloarthritis Program in the Division of Rheumatology.
Jean Liew, MD, MS, is an assistant professor of medicine at Boston University School of Medicine and conducts clinical research in osteoarthritis, axial spondyloarthritis and the intersection of COVID-19 with rheumatic disease. As a member of the COVID-19 Global Rheumatology Alliance (GRA) and a member of its steering committee, she is involved in multiple projects relating to data collection, analysis and dissemination of the impact of the COVID-19 pandemic on individuals with rheumatic disease.
Q&A
TR: How do the treatment recommendations differ for axSpA vs. non-radiographic axial spondyloarthritis (nr-axSpA)?

Dr. Gensler
Dr. Gensler: We consider this one disease under the broader, and probably more reliable, term, axial spondyloarthritis (axSpA). The bottom line is the treatment recommendations are the same. The distinction between radiographic axSpA and nr-axSpA is a gray area, technically differentiated by the burden of sacroiliac joint damage (i.e., grade 2 bilaterally or grade 3 or 4 unilaterally) on radiograph. I think the controversy arises for nr-axSpA because these patients have only been studied in trials in the presence of an objective sign of inflammation (e.g., magnetic resonance imaging [MRI] with bone marrow edema and/or elevated C-reactive protein [CRP]). These features may predict response to treatment, so when absent, one should pause and consider whether inflammation is driving disease activity.
There are some non-U.S. Food & Drug Administration (FDA) regulatory agencies that require MRI or CRP elevation in nr-axSpA to receive biologic treatment. Although I do not believe a positive MRI (with bone marrow edema) or elevated CRP is required for treatment, a negative MRI that also has no structural change of the sacroiliac joints—no erosions or subchondral fat—is concerning for the wrong diagnosis, and those patients should be treated with caution.
Dr. Hwang: In terms of axSpA, there is no significant difference in treatment recommendations between axSpA and nr-axSpA to my knowledge. Multiple studies show that regardless of radiographic status, there is a similar severity of disease activity and impact on physical function/health-related quality of life unless there is significant spinal disease. At this time, our current treatment guidelines from the major rheumatology organizations are based on disease activity.
Dr. Liew: For clinical practice, I would say the treatment recommendations (i.e., 2019 ACR/Spondyloarthritis Research and Treatment Network [SPARTAN]/Spondylitis Association of America [SAA] guidelines) don’t differ in terms of what you would actually do. What’s different is the level of evidence: Classification criteria established to identify an nr-axSpA population for studies were not established until about a decade ago. In both radiographic axSpA and nr-axSpA, non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line therapy, and in those who still have active disease despite NSAIDs, then the recommendation is to use tumor necrosis factor (TNF) inhibitors over other biologics.
TR: What are the most common mistakes you see in the management of axSpA?
Dr. Gensler: Hindsight is 20/20, and as healthcare professionals we all approach patients with the same ethos—to try to heal. When I see patients for second opinions, the most common reason the patient is still not doing well is that the diagnosis is not axSpA. I do see patients switched from one biologic to another for pain that is not necessarily driven by ongoing inflammation.

Dr. Hwang
Dr. Hwang: The diagnosis of axSpA can be very challenging. I think that classification criteria are sometimes misused for diagnostic purposes instead of their more intended purpose of creating a relatively homogenous population for research. For our fellows-in-training, I try to frame classification criteria as part of the scaffold to build the clinical acumen/diagnostic skills off of instead of the definitive answer.
On the other hand, we also see some patients in our spondyloarthritis clinic who carry an axSpA diagnosis—I believe with very reasonable suspicion in that initial evaluation—that have different rheumatic and other health conditions that can explain their symptoms. I believe this is, in part, due to anchoring bias that we all struggle with due to the mutual respect we have in the rheumatology field for our colleagues. I think a constant reevaluation of the underlying diagnosis is important, especially when patients have had lacked continuity of care.
Dr. Liew: For non-rheumatologists, including primary care providers, it’s really an issue of education and understanding of what axSpA is.
Even the terminology itself is confusing. And, like other diseases in rheumatology, there are no diagnostic criteria. There are classification criteria, which are meant to be specific enough to get a homogenous population for a clinical trial or an observational cohort. However, this is not something that is widely understood, so classification criteria are often incorrectly used for diagnosis.
Then, if you have someone who is unaware of nr-axSpA or of the terminology, perhaps they look at the older, modified New York criteria for AS, which require clear structural changes in the sacroiliac joints on plain films. This will, of course, miss everyone who has, or might have, nr-axSpA.
All of this contributes to the very long diagnostic delay. There have been many studies about this, performed over two or so decades and in multiple countries. Steven Zhao in the U.K. performed a systematic review and meta-analysis of these studies; the pooled mean diagnostic delay was nearly seven years.2 In addition, these studies have also shown that the diagnostic delay has persisted despite advances in understanding of the disease, and this leads to a treatment delay. And a treatment delay may lead to lower response to treatment when it’s finally initiated.
Again, it’s an education issue. But rheumatologists are not exempt from making mistakes with diagnosis and treatment in axSpA. And then you need to think: If it’s so difficult for us, how should we expect non-rheumatologists to get it right? There are people studying referral pathways to get patients with low back pain seen by rheumatologists sooner. For example, Fabian Proft in Germany published on the use of an online self-referral tool compared to a physician referral tool for chronic low back pain.3 In the U.S., a group at Yale is doing something similar with the Finding axSpA study (FaxSpA).4 Other groups are looking at improving referrals from ophthalmology, dermatology and gastroenterology clinics, for patients who have anterior uveitis, psoriasis or inflammatory bowel disease.
Other common mistakes, in no particular order:
- Not considering the diagnosis of axSpA in women. We learn that axSpA is a male-predominant disease, with an illness script of a white man in his 20s with inflammatory back pain that responds to NSAIDs and exercise and worsens with rest. Although the illness script still works, we need to consider who we’re missing if we focus on that demographic. In nr-axSpA we don’t see this male predominance, unlike in axSpA.
- Misdiagnosis based on imaging. I’ve discussed the problem of missing nr-axSpA due to reliance on radiographs. There is also the other issue: overdiagnosis. As a field, we are working on better definitions for features on MRI that would be consistent with a diagnosis of nr-axSpA vs. features that are non-specific, or are non-specific if not present with other abnormalities. Studies of athletes and postpartum women have shown that bone marrow edema on MRI of the sacroiliac joints can be seen in people without axSpA.5,6
- Not starting TNF inhibitors sooner. Coupled with this, the reliance on methotrexate and sulfasalazine for peripheral disease but sometimes also axial disease (methotrexate is not recommended for either; sulfasalazine is only in the recommendations for peripheral involvement). AxSpA should not be treated like rheumatoid arthritis—they are very different diseases.
- Not emphasizing physical therapy and exercise (of any type). These are recommended for all patients with axSpA but physical therapy is very underutilized. Of course, not every patient has good access or the time or other means to attend regularly.
TR: What are some tips for the clinical management of axSpA?
Dr. Gensler:
a) Make sure the diagnosis is correct. If you are in the possible/probable zone, then set a time frame for response to treatment and don’t anchor to a diagnosis if treatment response is in the placebo range or less.
b) Consider using data to drive your decisions. Because the physical exam for axial involvement is limited in early disease and slowly responsive to treatment, the peripheral exam is not typically abnormal and the inflammatory markers are often normal (especially in women), consider measuring disease activity. I use a Bath Anklylosing Spondylitis Disease Activity Index (BASDAI) as a point of care assessment and prefer an Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP) if the CRP is available. This gives me an outcome to look at over time that though subjective, compares to the patient’s own quantitative assessment from before. The ASDAS is almost always consistent with how the patient feels.
c) When patients come in complaining of increased pain or symptoms suggestive of disease activity, delve into both risks for uncontrolled disease (e.g., missed medications, changes in exercise, weight gain, stress, mental health) before switching to another medication. We have limited therapeutic options, so making sure the patient doesn’t have some other reversible reason for treatment failure is important. Additionally, if the reason for disease activity is not uncontrolled inflammation, then there is a risk of what I call the biologic spiral—switching from one drug to another without gaining disease control.
d) When sitting with a patient on a biologic with uncertain disease activity, consider using objective data to help drive decisions. Is the CRP elevated again? If not, do you need an MRI to identify bone marrow edema reflective of active disease, before changing?
e) Assess for extra-musculoskeletal manifestations as you consider treatment or treatment changes. I have a low threshold to evaluate for inflammatory bowel disease in axSpA.
Dr. Hwang: Structured exercise is very important. A balance of flexibility, postural, strength and aerobic exercises is important in the care of these patients. A trial of NSAIDs—as short as two to four weeks—can also be an alternative at times, before changing biologics, if patients have a partial response to biologic pharmacotherapy.

Dr. Liew
Dr. Liew: Patient education and empowerment are important. Curate helpful and reputable patient-directed resources. These can be found on the SAA website, www.spondylitis.org. Creaky Joints (www.creakyjoints.org) is another great resource, with educational materials and news articles vetted by clinicians.
Consider the comorbidities in your patients. I think we’ve gotten better at considering comorbidities in lupus, for example. Comorbidities are important for the care of axSpA as well. Think about cardiovascular disease, especially screening for risk factors such as hypertension (very common), hyperlipidemia and diabetes. Think about depression. Think about poor sleep.
Continue to emphasize physical therapy and exercise. There are continued benefits for inflammation and disease activity even in those with disease that is fairly well controlled on biologic therapy. There are educational materials available online, including videos for exercises and stretches that patients can do at home, from the U.K.-based National Axial Spondyloarthritis Society (NASS).
TR: What if you can’t get the insurer to authorize a treatment for nr-axSpA because it’s only approved for axSpA?
Dr. Gensler: Well, I don’t use the nr-axSpA diagnostic code, especially as we think about this blurry line that distinguishes non-radiographic from radiographic disease. I also don’t use ‘AS’ unless it is absolutely clear/classic as patients may then struggle to get disability/life insurance. I am able to use an ‘axial spondyloarthritis’ code in our EPIC electronic health record system, and I have never had a problem with a biologic based on these terms. Nr-axSpA is an indication, but only for a few specified biologics. I might still get denials, but a brief peer-to-peer conversation almost always resolves the denial.
Dr. Hwang: We understand that almost 20% of axSpA patients have peripheral symptoms. An emphasis on that aspect at times can be helpful. Also, because nr-axSpA is part of the continuum of axSpA, I don’t view these as separate diseases.
Dr. Liew: This was more of an issue before the FDA approved certolizumab pegol for nr-axSpA. Also, before there was a new International Classification of Diseases (ICD) code for nr-axSpA. Before this, people would code everything as ‘AS’ so that patients could get MRIs and could get biologic therapies. And this is fine and shouldn’t impact clinical care—but it does impact observational research using administrative or claims data where only ICD codes are available for diagnoses. It makes it hard for researchers to use these data to study AS or nr-axSpA as separate populations. (Brief epidemiology lesson: This study design issue is called misclassification.)
TR: We would love to hear your final thoughts. Any important pearls or points of interest to share with rheumatology professionals?
Dr. Gensler: AxSpA patients used to be easy to diagnose; the disease is now [recognized as] more heterogeneous and, therefore, more challenging to identify correctly, with many mimics to consider. Patients often come to us after years of disease. They know their disease, and this is often more comfortable than the unknown medications we are offering. When treating these patients, consider the non-pharmacologic interventions as similarly important as the medications we prescribe. It takes me several visits to build a therapeutic alliance before they are willing to consider escalation from NSAIDs. This is less of an issue in patients with short disease duration. Finally, what a joy to care for a group of patients that present to us in their 20s and 30s—we get to share in their many life milestones. The depth of those relationships is priceless.
Dr. Hwang: While it has its own struggles, I have really enjoyed co-managing my axSpA patients with other disciplines and services, specifically but not limited to dermatology, physical therapy and ophthalmology. This has allowed me to glean better understanding of their specialties and I believe our synergy has allowed us to improve our patients’ lives.
Dr. Liew: For trainees interested in axSpA or who just want to learn more, SPARTAN has an annual meeting in May with a full day dedicated to a trainee symposium. I’ve attended this symposium either live or virtually every year since 2018 and I’ve gotten something out of it each time. SPARTAN is committed to educating trainees, as well as supporting research and educational efforts. There are research grant opportunities to fellows, we are doing more educational outreach beyond rheumatology, and we are thinking of ways to connect fellows with mentors outside of their own institutions. Visit the website (www.spartangroup.org) or follow on Twitter @SPARTAN_Updates.
‘The distinction of radiographic axSpA & nr-axSpA is a gray area, technically differentiated by the burden of sacroiliac joint damage on radiograph.’ —Lianne S. Gensler, MD
It’s been a tough year with the pandemic, with many changes to the practice of rheumatology and with restrictions on in-person meetings (among other things). A bright light has been the expansion of virtual learning, as well as virtual platforms for seminars and webinars. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and SPARTAN put out a series of monthly lectures to cover clinical practice and current research in axSpA and psoriatic arthritis. These videos are available for viewing for free.
THE DRUGS
Note: For all drugs discussed, refer to the company websites for the full prescribing information and all Important Safety Information (ISI).
*Important Safety Information*
This ISI is applicable to all tumor necrosis factor inhibitors (TNFi’s) and some other biologic agents.
Serious Infections & Malignancies
- There is an increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (e.g., histoplasmosis) and infections due to other opportunistic pathogens. If these develop, discontinue the drug.
- Patients should be tested for latent TB prior to starting the drug and during therapy. Treatment for latent TB should be initiated prior to starting the drug. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with the drug, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent TB is needed prior to initiating the drug, even for patients previously vaccinated with Bacillus Calmette-Guerin (BCG).
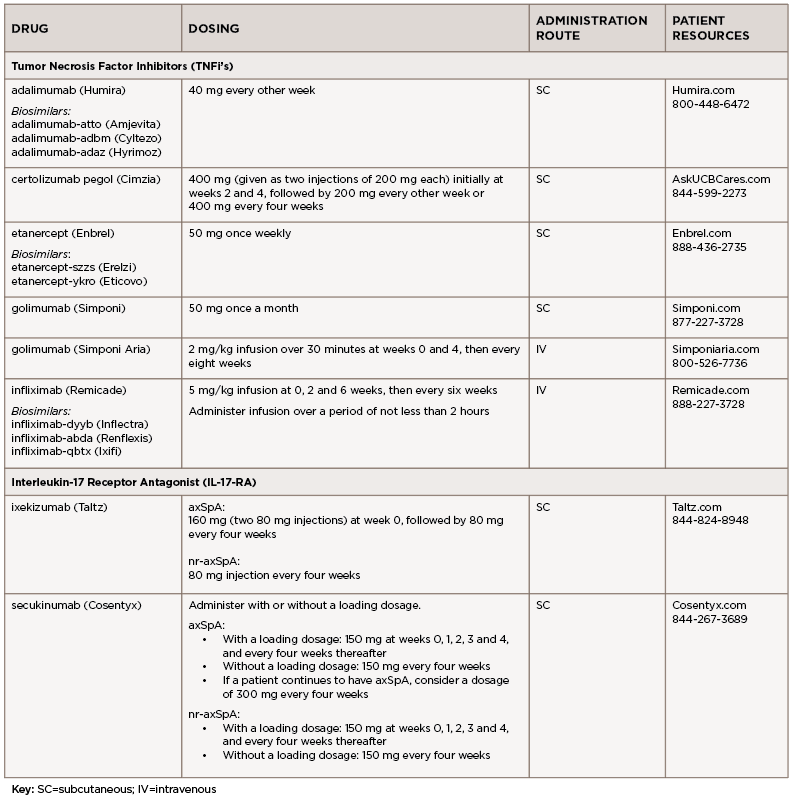
Adalimumab (Humira):7 injection
Biosimilars: adalimumab-atto (Amjevita),8 adalimumab-adbm (Cyltezo),9 adalimumab-adaz (Hyrimoz)10
Drug class: TNFi
Boxed Warning: Refer to important safety information (*ISI*) below and
- Fatal hepatosplenic T cell lymphomas (HSTCLs) have occurred post-marketing in adolescent and young adult inflammatory bowel disease (IBD) patients treated with TNFi’s.
Warnings & Precautions
- Do not start adalimumab during an active infection. If an infection develops, monitor carefully and stop adalimumab if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on adalimumab, consider empiric antifungal therapy for those at high risk who reside in, or travel to, regions where mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis, are endemic.
- The risks and benefits of treatment with TNFi’s, including adalimumab, should be considered prior to initiating therapy in patients with a known malignancy, other than a successfully treated non-melanoma skin cancer, or when considering continuing a TNF blocker in patients who develop a malignancy.
- Anaphylaxis or serious allergic reactions may occur.
- Hepatitis B virus (HBV) reactivation can occur. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop adalimumab and begin antiviral therapy.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cytopenias, pancytopenia—advise patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on adalimumab. Consider discontinuation of therapy in patients with confirmed significant hematologic abnormalities.
- Heart failure, worsening or new onset, may occur.
- Lupus-like syndrome—stop adalimumab if the syndrome develops.
Dosage & Administration
Adalimumab is administered by subcutaneous (SC) injection. The recommended dose is
40 mg every other week.
Commentary: The approval of adalimumab was based on two randomized, multicenter, double-blind, placebo-controlled clinical studies in close to 500 patients who had active axSpA. The results showed the drug was effective in reducing pain and inflammation in axSpA patients after 12 weeks of treatment, meeting the trial’s end point.
Certolizumab pegol (Cimzia):11 injection
Drug class: TNFi
Boxed Warning: Refer to *ISI* (left)
Warnings & Precautions
- Do not start certolizumab pegol during an active infection. If an infection develops, monitor carefully and stop the drug if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on certolizumab pegol, consider empiric antifungal therapy for those at high risk who reside in, or travel to, regions where mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis, are endemic.
- Cases of lymphoma and other malignancies have been observed in patients receiving TNFi’s.
- Heart failure, worsening or new onset, may occur.
- Anaphylaxis or serious allergic reactions may occur.
- HBV reactivation can occur; test for HBV infection before starting certolizumab pegol. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop the drug and begin antiviral therapy.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cytopenias, pancytopenia—advise patients to seek immediate medical attention if symptoms develop, and consider stopping certolizumab pegol.
- Avoid concomitant use with anakinra, abatacept, rituximab and natalizumab due to an increased risk of serious infections.
- Lupus-like syndrome—stop certolizumab pegol if the syndrome develops.
- Avoid live vaccines.
Dosage & Administration
AxSpA and nr-axSpA: Certolizumab pegol is administered by SC injection. The recommended dose is 400 mg (given as two SC injections of 200 mg each) initially at weeks 2 and 4, followed by 200 mg every other week or 400 mg every four weeks.
Commentary: Certolizumab pegol is the first FDA-approved treatment for nr-axSpA. The efficacy of certolizumab pegol for the treatment of nr-axSpA was studied in a randomized clinical trial of 317 adult patients with nr-axSpA and objective signs of inflammation, indicated by elevated C-reactive protein (CRP) levels and/or sacroiliitis on MRI. Improvement responses were greater for patients treated with certolizumab pegol than patients who received a placebo. The overall safety profile observed in the certolizumab pegol treatment group was consistent with the drug’s known safety profile.
Etanercept (Enbrel):12 injection
Biosimilars: etanercept-szzs (Erelzi),13 etanercept-ykro (Eticovo)14
Drug class: TNFi
Boxed Warning: Refer to *ISI* (p. 12)
Warnings & Precautions
- Do not start etanercept during an active infection. If an infection develops, monitor carefully and stop etanercept if the infection becomes serious.
- Consider empiric antifungal therapy for patients at high risk (those who reside in, or travel to, regions where mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis, are endemic) of invasive fungal infections who develop a severe systemic illness on etanercept.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cases of lymphoma have been observed in patients receiving TNFi’s.
- Congestive heart failure, worsening or new onset, may occur.
- Advise patients to seek immediate medical attention if symptoms of pancytopenia or aplastic anemia develop, and consider stopping etanercept.
- Monitor HBV carriers for reactivation during and following therapy. If reactivation occurs, consider stopping etanercept and beginning antiviral therapy.
- Anaphylaxis or serious allergic reactions may occur.
- Stop etanercept if lupus-like syndrome or autoimmune hepatitis develops.
Dosage & Administration
Etanercept is administered by SC injection. The recommended initial dose is 50 mg once weekly.
Commentary: Etanercept was the first biologic approved by the FDA to reduce the signs and symptoms in patients with axSpA. In a pivotal phase 3 study of 277 patients, 60% of etanercept-treated patients achieved a 20% improvement in the Assessment in Ankylosing Spondylitis Response Criteria (ASAS 20), a composite measure that includes back pain, morning stiffness, inflammation and physical function, compared with 27% of patients receiving placebo, after 12 weeks. The adverse events were similar to those reported in previous clinical trials, with injection site reactions occurring more frequently than in the placebo group.
Golimumab (Simponi):15 injection
Drug class: TNFi
Boxed Warning: Refer to *ISI* (p. 12)
Warnings & Precautions
- Do not start golimumab during an active infection. If an infection develops, monitor carefully and stop golimumab if the infection becomes serious.
- For patients who develop a systemic illness on golimumab, consider empiric antifungal therapy for those who reside in, or travel to, regions where mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis, are endemic.
- HBV many occur. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop golimumab and begin antiviral therapy.
- The incidence of lymphoma was seen more often than in the general U.S. population. Cases of other malignancies have been observed among patients receiving a TNFi.
- Heart failure, worsening or new onset, may occur. Stop golimumab if new or worsening symptoms occur.
- Demyelinating disease, exacerbation or new onset, may occur.
- Serious systemic hypersensitivity reactions including anaphylaxis may occur.
- Lupus-like syndrome—stop golimumab if the syndrome develops.
Dosage & Administration
Golimumab is administered by SC injection. The recommended dose is 50 mg once a month.
Commentary: The FDA approval of golimumab injection was based on the results of a phase 3 clinical trial that enrolled 356 patients. The most common adverse reactions (>5%) were upper respiratory tract infection, nasopharyngitis and injection site reactions.
Golimumab (Simponi Aria):16 infusion
Drug class: TNFi
Boxed Warning: Refer to *ISI* (p. 12)
Warnings & Precautions: Refer to *Simponi
Dosage & Administration
Golimumab is administered by intravenous (IV) infusion. The recommended dose is 2 mg/kg over 30 minutes at weeks 0 and 4, then every eight weeks.
Commentary: The FDA approval of golimumab injection for IV use was based on results from two clinical trials that enrolled more than 600 patients. The trial showed the drug significantly improved the signs and symptoms of axSpA. The most common adverse reactions (>5%) were upper respiratory tract infection, nasopharyngitis and injection site reactions.
Infliximab (Remicade):17 infusion
Biosimilar(s): infliximab-dyyb (Inflectra),18 infliximab-abda (Renflexis),19 infliximab‑qbtx (Ixifi)20
Drug class: TNFi
Boxed Warning: Refer to *ISI* (p. 12) and
• Fatal hepatosplenic T cell lymphoma (HSTCL) have been reported in patients treated with TNFi’s, post-marketing. All infliximab cases occurred in inflammatory bowel disease (IBD) patients, who were mostly adolescent or young adult males. All had received azathioprine or 6-mercaptopurine concomitantly with infliximab at, or prior to, diagnosis.
Warnings & Precautions
- Do not give infliximab during an active infection. If an infection develops, monitor carefully and stop infliximab if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on infliximab, consider empiric antifungal therapy for those who reside or travel to regions where mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis, are endemic.
- The incidence of malignancies, including lymphoma, was greater in infliximab treated patients than in controls. Due to the risk of HSTCL, carefully assess the risk/benefit especially in patients with IBD, men and in patients receiving azathioprine or 6-mercaptopurine treatment.
- HBV reactivation can occur. Therefore, test for HBV infection before starting infliximab. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop infliximab and begin antiviral therapy.
- Hepatotoxicity—rare, severe hepatic reactions, some fatal or necessitating liver transplantation, have occurred. Stop infliximab in the presence of jaundice and/or marked liver enzyme elevations.
- Heart failure, new onset or worsening, may occur.
- Cytopenias—advise patients to seek immediate medical attention if signs and symptoms develop, and consider stopping infliximab.
- Hypersensitivity—serious infusion reactions including anaphylaxis or serum sickness-like reactions may occur.
- Demyelinating disease, exacerbation or new onset, may occur.
- Lupus-like syndrome—stop infliximab if syndrome develops.
- Live vaccines or therapeutic infectious agents should not be given with infliximab.
Dosage & Administration
Infliximab is administered by IV infusion over a period of not less than two hours. The recommended dose is 5 mg/kg infusion at 0, 2 and 6 weeks, then every six weeks.
Commentary: The FDA approved infliximab for treatment of axSpA was based on data from a phase 3 clinical trial that enrolled 279 patients. The most common adverse reactions (≥10%) were infections (e.g., upper respiratory, sinusitis, pharyngitis), infusion-related reactions, headache and abdominal pain.
Ixekizumab (Taltz):21 injection
Drug class: IL-17-RA
Warnings & Precautions
- Serious infections have occurred. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue ixekizumab until the infection resolves.
- Evaluate for TB prior to initiating treatment.
- If a serious hypersensitivity reaction occurs, discontinue ixekizumab immediately and initiate appropriate therapy.
- IBD, including exacerbations, occurred during clinical trials. Monitor closely patients who are treated with ixekizumab and have IBD.
Dosage & Administration
Ixekizumab is administered by subcutaneous injection.
- axSpA: The recommended dose is 160 mg (two 80 mg injections) at week 0, followed by 80 mg every four weeks.
- nr-axSpA: The recommended dose is 80 mg injection every four weeks.
Commentary: The FDA approval of ixekizumab for axSpA was based on two randomized, controlled phase 3 clinical trials that included a total of 657 adult patients. The most common adverse reactions (≥1%) were injection-site reactions, upper respiratory tract infections, nausea and tinea infections.
Secukinumab (Cosentyx):22 injection
Drug class: IL-17-RA
Warnings & Precautions
- Serious infections have occurred. Caution should be exercised when considering using secukinumab in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue the drug until the infection resolves.
- Prior to initiating treatment with secukinumab, evaluate for TB.
- Caution should be exercised when prescribing secukinumab to patients with IBD; cases of IBD were observed in clinical trials.
- If an anaphylactic reaction or other serious hypersensitivity reaction occurs, discontinue secukinumab immediately and initiate appropriate therapy.
Dosage & Administration
Secukinumab is administered by subcutaneous injection. Administer with or without a loading dosage.
axSpA:
- With a loading dosage: 150 mg at weeks 0, 1, 2, 3 and 4, and every four weeks thereafter;
- Without a loading dosage: 150 mg every four weeks;
- If a patient continues to have active axSpA, consider a dosage of 300 mg every four weeks.
nr-axSpA:
- With a loading dosage: 150 mg at weeks 0, 1, 2, 3 and 4, and every four weeks thereafter;
- Without a loading dosage: 150 mg every four weeks.
Commentary: The FDA approval of secukinumab for axSpA was based on four randomized, controlled phase 3 clinical trials that included a total of 1,500 adult patients. The most common adverse reactions (≥1%) were nasopharyngitis, diarrhea and upper respiratory tract infection.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. Dr. Choy is director of pharmacy practice at the New York State Council of Health-system Pharmacists. She is also the coauthor of Healthcare Heroes: The Medical Careers Guide.
References
- Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019 Oct; 71(10):1599–1613.
- Zhao SS, Pittam B, Harrison NL, et al. Diagnostic delay in axial spondyloarthritis: A systematic review and meta-analysis. Rheumatology (Oxford). 2021 Apr 6;
60(4):1620–1628.
- Proft F, Spiller L, Redeker I, et al. Comparison of an online self-referral tool with a physician-based referral strategy for early recognition of patients with a high probability of axial SpA. Semin Arthritis Rheum. 2020 Oct;50(5):1015–1021.
- Afinogenova Y, Alexander S, Haims A, et al. Use of Facebook and electronic patient portal to identify axial spondyloarthritis in patients with chronic back pain. ACR meeting abstract #1894. 9 Nov 2020.
- Weber U, Jurik AG, Zejden, et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: Exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 2018 May;70(50):736–745.
- Baraliakos X, Richter A, Feldmann D, et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann Rheum Dis. 2020 Feb;79(2):186–192.
- FDA website. Humira prescribing information. 2021 Feb.
- FDA website. Amjevita prescribing information. 2019 Jun.
- FDA website. Cyltezo prescribing information. 2019 Sep.
- FDA website. Hyrimoz prescribing information. 2021 Feb.
- FDA website. Cimzia prescribing information. 2019 Sep.
- FDA website. Enbrel prescribing information. 2020 Aug.
- FDA website. Erelzi prescribing information. 2020 Jun.
- FDA website. Eticovo prescribing information. 2019 Apr.
- FDA website. Simponi prescribing information. 2019 Sep.
- FDA website. Simponi Aria prescribing information. 2021 Feb.
- FDA website. Remicade prescribing information. 2020 May.
- FDA website. Inflectra prescribing information. 2021 Jun.
- FDA website. Renflexis prescribing information. 2019 Jun.
- FDA website. Ixifi prescribing information. 2020 Jan.
- FDA website. Taltz prescribing information. 2021 Mar.
- FDA website. Cosentyx prescribing information. 2021 May. . 2021 Jul. https://www.niams.nih.gov/grants-funding/funding-opportunities/diversity-supplement-guidelines.