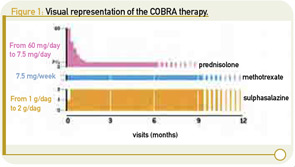
At present, there are many different ways to treat rheumatoid arthritis (RA), raising questions about which approaches produce the best results in the real-world setting and which are the most cost effective. Among these approaches, COBRA (based on the Dutch acronym Combinatie therapie Bij Rheumatoide Arthritis) therapy is a well-known and thoroughly investigated combination therapy for the treatment of early RA. This combination consists of three disease-modifying antirheumatic drugs (DMARDs), including methotrexate, sulfasalazine, and an initial period with a high dose of prednisolone (60 mg/d tapered in six weeks to 7.5 mg/d). (See Figure 1, p. 23.) COBRA leads to faster suppression of disease activity and less joint damage in the long term compared with sulfasalazine monotherapy. Moreover, the COBRA combination is as effective as high-dose methotrexate in combination with infliximab and is cost effective compared with other antirheumatic therapies.1-5
Despite these features and its long-term benefits, COBRA therapy is not often prescribed in clinical practice, at least not by Dutch rheumatologists. We therefore asked, “Why not?” and performed studies to better understand the prescription pattern of COBRA and determine whether it can be changed. For our study, we sent a small questionnaire to every rheumatologist in the Netherlands to measure attitudes towards the combination therapy and the frequency of use.
From the responses, we learned that COBRA therapy was regarded as effective and safe but also rather complicated to administer. Remarkably, although the overall attitude of rheumatologists towards COBRA therapy was slightly positive, the majority of responding rheumatologists did not intend to prescribe COBRA therapy in the near future. These results gave us the first indication of the contradictory feelings surrounding COBRA therapy.6
To study this discrepancy further, we organized focus group discussions with both patients and rheumatologists (in separate groups). In these groups, rheumatologists indicated that they were positive about the effectiveness of COBRA therapy but concerned about their patients’ possible negative reaction to the large number of pills to be taken. Doctors feared the reaction of patients, including a sense of failure toward their providers and that the therapy prescribed is considered disagreeable. In addition, rheumatologists felt that they lacked the time to explain and prescribe COBRA therapy and felt uncomfortable prescribing high doses of prednisolone.7
On the other hand, patients who joined the focus groups (mostly late RA patients with a broad experience of treatment regimens, including COBRA) were positive about an aggressive combination therapy such as COBRA therapy. Importantly, patients said that they had no qualms taking many pills if this would provide immediate relief of their symptoms or might improve their prognosis. Patients associated prednisolone with negative side effects (especially their effects on appearance), but they were also aware of its benefits and the need to take prednisolone in rough times of active arthritis. Patients stated that they based their opinions on their own experience at the start of the disease and concluded that taking many pills felt much better than the pain and limitations they were facing with the disease. A decrease in the number of pills after a period of intensive treatment was highly appreciated.
From these focus groups, it appears that the rheumatologist is more afraid of COBRA therapy than the patient is and that the low prescription rate is mostly determined by the opinion of the rheumatologist.
11-Year Safety Data
Because rheumatologists expressed worries about the long-term side effects of the initial high dose of prednisolone, we performed a follow-up study to the COBRA trial.8 This study revealed that there were no real differences in long-term side effects between the two treatment groups (combination versus sulfasalazine) that could be contributed to the initial study drugs. Mortality in the COBRA group was numerically lower, and cardiovascular and other major comorbidities were similar to that in the sulfasalazine group. The prevalence of hypertension and diabetes was increased in the COBRA group but offset by a decrease in hypercholesterolemia. Prevalence of osteoporosis was also comparable. Moreover, at the discretion of the treating physician, if COBRA is continued for 10 years of therapy, the benefits on the disease itself and reduction in damage progression are maintained; these benefits may even continue to increase after COBRA therapy, depending on how one deals with the selective drop-out.8
Testing an Implementation Plan
After we evaluated all aspects of COBRA therapy from the patient’s and rheumatologist’s point of view, as well as the long-term effects on disease progression, we designed an implementation strategy.9 Our central objective was to make it easier and more agreeable for rheumatologists, patients, and arthritis nurses to use COBRA therapy in clinical practice. Many clues for improvement of daily use by rheumatologists as well as patients were extracted from the rich data gathered during focus group discussions.
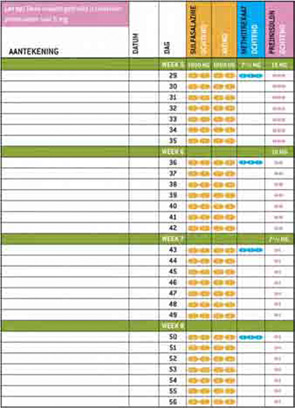
As we learned in our survey and focus group, rheumatologists felt that COBRA therapy is complicated and takes a lot of time to prescribe. Furthermore, they felt that patients might object to the use of a large number of pills, including prednisolone. To remove the barrier of workload and provision of tailored information, we developed a patient information booklet, including visual aids to explain the therapy, clear tables on the intake of the different drugs, and preprinted prescriptions to speed up the prescribing process. (See Figure 2, at right.) We also disseminated the results of the patient focus groups, in which patients explained that the temporary use of a large amount of pills or prednisolone was acceptable, as long as this would take away their immediate complaints and improve their prognosis.
To further improve patient comfort while using COBRA therapy, the patient information material also contained a written interview in which a fictional early RA patient explained her feelings during diagnosis and the start of COBRA therapy. The content of this interview was also derived from the focus group discussions with patients and was used in the booklet to give patients a positive boost when starting this therapy. We provided a group of rheumatologists and arthritis nurses with this implementation material and asked them to prescribe COBRA therapy to their patients using the supplied material. All parties involved—rheumatologists, patients, and nurses—evaluated the material and gave positive responses. The website, which features the patient information booklet as well as all the scientific research around COBRA therapy presented at congresses and in articles is currently online at www.cobratherapy.nl. With this pilot study, the material is ready to be implemented in clinical practices around the world.
The Future of COBRA Therapy
Our findings and clinical experience indicate that COBRA therapy is effective, safe, and—with the proper education materials—easy to use in clinical practice by patients as well as rheumatologists and arthritis nurses. Moreover, worldwide implementation of COBRA therapy as a first choice for newly diagnosed RA patients with an aggressive arthritis makes sense, not only from a medical perspective, but perhaps also from an economic one and a patient’s point of view. Because COBRA therapy is a combination of inexpensive, generic drugs, it is a highly cost-effective treatment of early RA. Patients would benefit from implementation of COBRA therapy because this therapy gives them a better prognosis than DMARD monotherapy.
We suggest that rheumatologists around the world strongly consider COBRA therapy for patients with active RA on first presentation. In case of failure to respond, patients can rapidly switch to a biological in combination with methotrexate, as the conditions set by some national authorities to have failed on at least two DMARDs have been met. This strategy ensures fast, effective, and cost-effective treatment for early active RA patients, increasing their chances to regain their quality of life and prevent long-term damage to their joints.
COBRA therapy is probably most often used in the Netherlands, where the COBRA trial was originally conducted (in cooperation with Leuven, Belgium). The Dutch website, which has been online for a year, is frequently visited. More recently, we learned that COBRA therapy has been implemented at the Bristol University Royal Infirmary in the U.K., where a group of the most severely affected early RA patients receive COBRA therapy as standard care.
We would like to receive your comments and experiences with COBRA therapy in your practice. Furthermore, if you are interested in using material to facilitate prescription of the therapy, we would be happy to help. This is an exciting time in rheumatology because of treatment advances. Choice is never easy, however, especially for a chronic disease like RA where treatment can be prolonged, expensive, and associated with unknown risks. We believe that our experience in implementing the use of COBRA is valuable and should be considered as the treatment options in the future increase.
Drs. van Tuyl and Boers work in the departments of rheumatology and epidemiology and biostatistics at VU University Medical Center in Amsterdam, the Netherlands.
References
- Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309-318.
- Landewé RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: Long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347-356.
- Goekoop-Ruiterman YP, De Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: A randomized trial. Ann Intern Med. 2007;146:406-415.
- Korthals-de Bos I, Van Tulder M, Boers M, et al. Indirect and total costs of early rheumatoid arthritis: A randomized comparison of combined step-down prednisolone, methotrexate, and sulfasalazine with sulfasalazine alone. J Rheumatol. 2004:31:1709-1716.
- Verhoeven AC, Bibo JC, Boers M, Engel GL, van der LS. Cost-effectiveness and cost-utility of combination therapy in early rheumatoid arthritis: Randomized comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone. COBRA Trial Group. Combinatietherapie Bij Reumatoide Artritis. Br J Rheumatol. 1998:37:1102-1109.
- van Tuyl LH, Plass AM, Lems WF, Voskuyl AE, Dijkmans BA, Boers M. Why are Dutch rheumatologists reluctant to use the COBRA treatment strategy in early rheumatoid arthritis? Ann Rheum Dis. 2007;66:974-976.
- van Tuyl LH, Plass AM, Lems WF, et al. Discordant perspectives of rheumatologists and patients on COBRA combination therapy in rheumatoid arthritis. Rheumatology. 2009;36:1380-1386.
- van Tuyl LH, Boers M, Lems WF, et al. Survival, comorbidities and joint damage 11 years after the COBRA combination therapy trial in early rheumatoid arthritis. Ann Rheum Dis. 2010;69:807-812.
- van Tuyl LH, Plass AM, Lems WF, et al. Facilitating the use of COBRA combination therapy in early rheumatoid arthritis: A pilot implementation study. J Rheumatol. 2009;36:1380-1386.