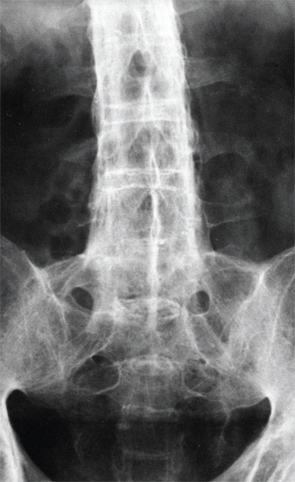
X-ray of the lower spine of a patient affected by ankylosing spondylitis. BSIP/Science Source
Understanding how human leukocyte antigen (HLA) class I molecule B27 promotes spondyloarthritis has intrigued researchers for four decades. Although the association between the single gene variant HLA-B27—specifically some of its subtypes—with ankylosing spondylitis (AS) is particularly strong, how HLA-B27 directly influences disease development has not yet been clearly explained, although hypotheses continue to be generated.
Results of recent research indicate that conformational flexibility—or the tendency of certain HLA-B27 subtypes to unfold or misfold—could be key to understanding why certain subtypes are associated with disease development, according to Robert A. Colbert, MD, PhD, co-author with Simon Powis, PhD, at the University of St. Andrews, UK, of an editorial in the May issue of Arthritis & Rheumatology.1,2
The inherent flexibility in the subtypes associated with disease “suggests that the proteins that are disease associated may behave differently,” says Dr. Colbert, chief of the Pediatric Translational Research Branch and deputy clinical director at the National Institute of Arthritis and Musculoskeletal and Skin Diseases. “There are some important differences between disease-associated and non-associated subtypes that may provide more clues about B27 disease,” he says.
HLA-B27 Associated Diseases
HLA-B27 is associated with development of spondyloarthritis, which affects approximately 0.6–1% of the adult U.S. population. The most common type, which is AS, affects about 0.3% of that population. In an article in the Annual Review of Immunology in 2015, Paul Bowness, MD, PhD, at Botnar Research Centre, University of Oxford, United Kingdom, says that roughly 94% of patients with AS are HLA-B27 positive and that B27 is the “most important genetic factor.”3 Even so, “it still only contributes about one-third of the total heritability of AS (which is remarkably high, at approximately 90%),” he says.
Dr. Bowness says that between 30% and 75% of patients with reactive arthritis are positive for HLA-B27. Also, 33–75% of patients with colitis-associated spondyloarthritis, 40–50% with psoriatic spondyloarthritis, 76% with juvenile enthesitis-related arthritis, and 50% with acute anterior uveitis are positive for HLA-B27. At least 25% of patients with AS develop anterior uveitis over their lifetime, Dr. Bowness reports, but the condition can occur without AS and is still associated with HLA-B27.
The prevalence of AS in different populations reflects the prevalence of HLA-B27, he says. There are more than 100 subtypes of HLA-B27, and the predominant subtypes differ between populations, such as among European, Thai and Indonesian populations, and a rare subtype has been found nearly exclusively in people of Sardinian descent, according to Dr. Bowness.
Testing for HLA-B27
For patients with the signs and symptoms of spondyloarthritis, the HLA-B27 genetic test can help pediatric and adult rheumatologists predict whether they will develop AS, Dr. Colbert explains. The presence or absence of HLA-B27 is also used to classify patients with different types of arthritis and is useful because HLA-B27 is “such a strong risk factor for developing arthritis in the sacroiliac joints and in the spine,” he says. “If a patient has signs and symptoms and you are thinking about the diagnosis, it can be a good predictor of whether someone will develop AS,” Dr. Colbert says.
However, HLA-B27 testing cannot be used to make a diagnosis of AS because not everyone with AS has HLA-B27, he says. It also cannot be used reliably to predict development of the disease among people who have no symptoms of arthritis, because only about 1–5% of people with HLA-B27 will ever develop spondyloarthritis.
Treatment of AS has improved with the use of TNF inhibitors, and other treatments are being tested. Biologics that block other cytokine pathways, such as the interleukin (IL) 23/IL-17 axis, show much promise for treatment of the disease, he says.
One of the toughest questions in rheumatology has been and continues to be how HLA-B27 promotes development of spondyloarthritis. Answering that question could eventually lead to new and better therapies.
“There are still major questions about how to treat the aberrant bone formation that is a major contributor to morbidity in AS,” Dr. Colbert says. “If we could understand more about this major genetic risk factor, HLA-B27, then we might be able to figure out even better ways to treat the disease.”
Family of Proteins
Several theories have driven research about the pathogenic role of HLA-B27 over the past 40 years.
HLA-B27 is part of a family of proteins that carries peptides to the cell surface, “displaying them on the cell surface as a signal to CD8+ T cells and NK cells in the immune system that everything is OK,” Dr. Colbert explains. When a cell is infected with a virus or perhaps certain bacteria, the proteins from the viruses and bacteria are displayed, and the immune system is able to distinguish those from the self-peptides and recognize that the cell is in trouble and needs to be destroyed.
One among the several hypotheses generated about HLA-B27 has been that it presents self-peptides from the cell that somehow triggers the immune system, making it attack cells in the joints and the spine. The hypothesis was that it was this ability to present arthritogenic peptides that was the problem, but that “doesn’t seem to be the right answer. It hasn’t been completely ruled out, but many studies suggest that this is not the problem in AS,” Dr. Colbert says.
Investigators have found that HLA-B27 has some aberrant features and has a tendency to fold improperly or misfold. Part of this entails dimerization of two molecules of HLA-B27, which “causes stress for the cell and the triggering of stress-relief pathways that send signals out to neighboring cells. Some of these signals may promote inflammation and may eventually lead to arthritis,” he says.
Dimerization of the HLA-B27 molecules also happens when HLA-B27 on the cell surface is “brought inside the cell for recycling. Normally, this process can lead to degradation, but sometimes for HLA-B27 when this happens, it actually forms dimers and they get back out on the cell surface. It has been shown that these dimers can trigger T cells—specifically CD4+ T cells—that are predisposed to make pro-inflammatory cytokines.
“Researchers are still trying to figure out if these mechanisms explain all or part of the HLA-B27 story,” he adds.
In the May issue of Arthritis & Rheumatology, Loll and colleagues published a very interesting observation, according to Dr. Colbert.1 Their research asked a different question, which was based on the understanding that not all HLA-B27 molecules predispose people to AS.
Many of the subtypes are similar, but not identical. In fact, two subtypes—B*27:06 and B*27:09—are either not strongly associated with or, perhaps for B*27:06, not at all associated with AS. However, two other common subtypes-B*27:05 and B*27:04-“clearly do increase your chances of having disease,” Dr. Colbert says.
There are more than 100 subtypes of HLA-B27, & the predominant subtypes differ between populations, such as among European, Thai & Indonesian populations, & a rare subtype has been found nearly exclusively in people of Sardinian descent, according to Dr. Bowness.
“Since B*27:06 and B*27:09 are very closely related to B*27:05 and B*27:04, researchers have thought for a long time that by comparing them very carefully, any differences might explain the differential disease association.” Previous studies have shown that the B*27:05 subtype could hold the same peptide in two different conformations, but not B*27:09, suggesting that the two conformations would promote autoreactivity and perhaps explain arthritogenicity.
The current research by Loll et al probed the differences between the subtypes and found that the dual peptide conformation theory “did not hold for B*27:06 and B*27:04, because the non-associated subtype B*27:06 held the same peptide in two conformations, while the disease-associated B*27:04 subtype had only one conformation.”
Using infrared spectroscopy, they looked at conformational flexibility and found that the disease-associated subtypes are “floppy or non-rigid, whereas the non-disease-associated subtypes are more rigid. This inherent flexibility may help to explain why the HLA-B27 protein behaves differently, has a tough time folding, and tends to unfold more readily, both of which may contribute to dimerization,” Dr. Colbert says.
Does This Change Therapy?
“The HLA-B27 story continues to unfold,” he says. Although these research results are intriguing, they don’t change how rheumatologists should use the test for HLA-B27, but they are “one step forward in the path toward understanding how HLA-B27 causes disease.”
The Loll et al research fits with the idea that the flexibility and unfolding and tendency to dimerize may be important to how HLA-B27 causes disease. Additionally, the results indicate that more research is needed to understand the implications of that to the cells that express HLA-B27, Dr. Colbert says.
“The biggest implication for therapy would be to understand how HLA-B27 contributes to this disease. In other words, can we prevent HLA-B27 from dimerizing? Can we fix a subtype associated with disease, cause it to fold better and not dimerize?” That is not yet a possibility, but researchers continue to investigate HLA-B27 and its aberrant features at several labs around the world.
Kathy L. Holliman, MEd, is a medical writer based in Beverly, Mass.
References
- Loll B, Fabian H, Huser H, et al. Increased conformational flexibility of HLA-B*27 subtypes associated with ankylosing spondylitis. Arthritis Rheumatol. 2016 May;68(5):1172–1182.
- Powis SJ, Colbert RA. Editorial: HLA-B27: The story continues to unfold. Arthritis Rheumatol. 2016 May;68(5):1057–1059.
- Bowness P. HLA-B27. Annu Rev Immunol. 2015;33:29–48.