This case emphasizes the need to consider AS in patients with ILD and undifferentiated connective tissue disease and to screen for ARS antibodies, particularly in the presence of typical clinical features (mechanic’s hands), a negative ANA and a positive anti-cytoplasmic signal. The severity of anti-PL-12 AS varies because of the constant pulmonary involvement. ILD is the predominant prognosis factor and is notable in cases associated with pulmonary hypertension.
Myositis-Related Autoantibodies
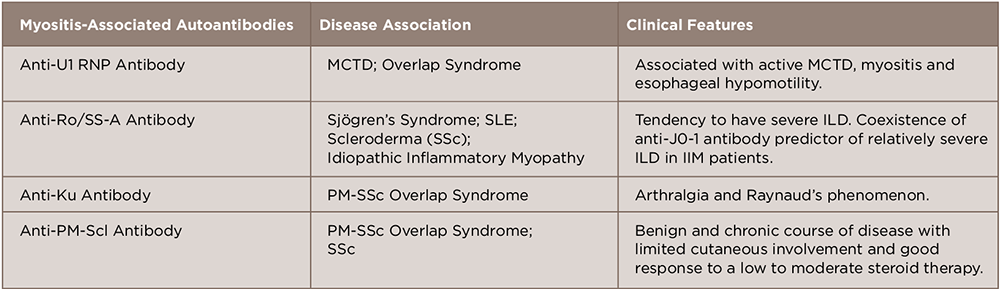
During the past decade, novel myositis-specific and myositis-associated autoantibodies have been identified that help us in the clinical diagnosis, classification, prediction of prognosis and choice of treatment in patients with idiopathic inflammatory myopathy (IIM).13
A number of antibodies detected in this disease can be subcategorized as myositis-specific and myositis-associated autoantibodies.14 These are closely associated with the clinical manifestations of polymyositis/dermatomyositis, such as symptoms, complications, reactivity to therapy and prognosis. Although considerable progress has been made in elucidating the association between genotype, serotype and clinical phenotype, further characterization of autoantigens and autoantibodies will help us understand the pathophysiology and provide us with a new therapeutic strategy in treating IIM (see Tables 1 and 2).
Conclusion
In conclusion, anti-PL-12-positive patients have a prevalence of ILD of 90–100%, with a high prevalence of isolated ILD at presentation, a lower frequency of myositis with a better response to therapy and an overall poorer prognosis compared with anti-Jo-1-positive patients.
 Quretul Quresh, MD, is a rheumatology fellow at Ochsner Medical Center, New Orleans. She completed her internal medicine residency at the University of Massachusetts School of Medicine, Berkshire Medical Center, Pittsfield, Mass. Her clinical and research interests are in the role of T cell homeostasis in rheumatoid arthritis and pathogenesis of PD1 pathway in inflammatory arthritis.
Quretul Quresh, MD, is a rheumatology fellow at Ochsner Medical Center, New Orleans. She completed her internal medicine residency at the University of Massachusetts School of Medicine, Berkshire Medical Center, Pittsfield, Mass. Her clinical and research interests are in the role of T cell homeostasis in rheumatoid arthritis and pathogenesis of PD1 pathway in inflammatory arthritis.
 Stephen Lindsey, MD, is a clinical professor of medicine at Louisiana State University (LSU) School of Medicine in New Orleans and a rheumatologist at Ochsner Medical Center. He is a graduate of LSU Medical School, did his internal medicine residency at Letterman Army Medical Center, San Francisco, and completed a rheumatology fellowship at Walter Reed Army Medical Center, Washington, D.C. His research interests include osteoporosis and the role of monoclonal antibodies and JAK inhibitors in rheumatoid arthritis.
Stephen Lindsey, MD, is a clinical professor of medicine at Louisiana State University (LSU) School of Medicine in New Orleans and a rheumatologist at Ochsner Medical Center. He is a graduate of LSU Medical School, did his internal medicine residency at Letterman Army Medical Center, San Francisco, and completed a rheumatology fellowship at Walter Reed Army Medical Center, Washington, D.C. His research interests include osteoporosis and the role of monoclonal antibodies and JAK inhibitors in rheumatoid arthritis.
References
- Kalluri M, Sahn SA, Oddis CV, et al. Clinical profile of Anti-PL2 autoantibody. Cohort study and review of the literature. Chest. 2009 Jun;135(6):1550–1556.
- Targoff IN. Autoantibodies and their significance in myositis. Curr Rheumatol Rep. 2008 Aug;10(4):333–340.
- Hervier B, Benveniste O. Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr Rheumatol Rep. 2013 Aug;15(8):349.
- Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmune Rev. 2014 Apr–May;13(4–5):367–371.
- Labirua-Iturburu A, Selva-O’Callaghan A, Vincze M, Danko K, et al. Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: Clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Medicine (Baltimore). 2012 Jul;91(4):206–211.
- Marie I, Josse S, Decaux O, et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med. 2013 Jul;24(5):474–479.
- Lega JC, Fabienn N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: A meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmune Rev. 2014 Sep;13(9):883–891.
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. 2014 Jan;73(1):227–232.
- Hervier B, Devilliers H, Stanciu R, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmune Rev. 2012 Dec;12(2):210–217.
- Marie I, Josse S, Decaux O, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmune Rev. 2012 Aug;11(10):739–745.
- Hervier B, Wallaert B, Hachulla E, et al. Clinical manifestations of anti-synthetase syndrome positive for anti-alanyl-tRNA synthetase (anti-PL12) antibodies: A retrospective study of 17 cases. Rheumatology (Oxford). 2010 May;49(5):972–976.
- Yoshifuji H, Fuji T, Kobayashi S, et al. Anti-aminoacyl tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006 May;39(3):233–253.
- Targoff IN. Immune manifestations of inflammatory muscle disease. Rheum Dis Clin North Am. 1994 Nov;20(4):857–880.
- Nakashima R, Mimori T. Clinical and pathophysiological significance of myositis-specific and myositis-associated autoantibodies. Int J Clin Rheumatol. 2010;5(5):523–536.