SAN FRANCISCO—A granulomatous vasculitis of the aorta and its branches, giant cell arteritis (GCA) can cause blindness, stroke and aortic aneurysm. GCA is a “frightening” disease, said Cornelia M. Weyand, MD, PhD, professor of medicine, and chief, Division of Immunology and Rheumatology at Stanford University School of Medicine, Stanford, Calif., during her presentation at the California Rheumatology Alliance’s 10th Annual Medical and Scientific Meeting. Using case examples and humorous analogy, Dr. Weyand shared guidance for diagnosing GCA, lessons learned about the inflammatory cascade involved in the disease and implications for treating patients who develop it.
The Right Diagnosis
Presenting symptoms of GCA aortitis can include pulse deficits, fever, a highly elevated sedimentation rate, aneurysm or aortic dissection. GCA can co-occur with polymyalgia rheumatica. Age older than 50 years is a predominant risk factor, said Dr. Weyand, and can help clinicians differentiate patients with GCA from those with Takayasu’s arteritis (TA), which occurs almost exclusively in patients younger than 40 years. In addition, TA almost always affects the carotid arteries and the proximal parts of the subclavian arteries, and frequently is seen in abdominal branches of the aorta; GCA has a preference for the distal subclavian and axillary arteries.
Radiology colleagues can be powerful allies during the diagnostic process. “CT angiography,” Dr. Weyand asserted, “gives us fast and very reliable data on whether patients have large vessel involvement. You need to work with your radiologist to be sure you look not only at the core of the aorta and the large vessels, but also into the periphery.” PET scans, she added, “are not as sensitive as we hoped they would be, particularly in patients that are on treatment.” There may be advances with new combined PET/MRI technology (Note: In January, Stanford installed the world’s first fully integrated PET/MRI scanner), but that has yet to be demonstrated.
Compared with men, women are at greater risk for pain undertreatment. “Maybe it’s because women exprThere is no evidence right now that if you were to stop the disease entirely that the prognosis of our patients would improve.
What Goes Wrong?
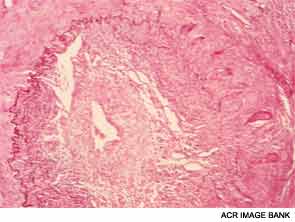
Dr. Weyand shared lessons learned from her research into the mechanisms of inflammatory blood vessel disease conducted with colleagues at both Stanford University and the Mayo Clinic in Rochester, Minn. In GCA, vascular inflammation results from a domino effect of activated cytokines, producing a sort of “cytokine soup.” Macrophages cohere to form granulomas, often within the medial layer of the aorta. The site of entrance for the inflammatory cells is the adventitia in the outer lining of the aorta, she pointed out. Analysis of cell lesions has demonstrated a complex makeup of two different T-cell populations in addition to three different dendritic cell populations. As opposed to atherosclerosis, in which lesions form in the interior of the blood vessel, in GCA the disease process starts “at the back door.”
Dr. Weyand’s research has demonstrated that the blood vessel itself makes an important contribution to the disease process. The vascular system, she stated, communicates with the immune system. “The vascular system actually works for the immune system. It’s like the NSA,” she noted wryly. “It collects information, but we’re not so sure where it puts it, and we’re not sure that there’s anybody that actually oversees this process,” she added, to laughter from the audience. “I can tell you that it’s the artery that actually makes the decision; it’s not the immune system. As an immunologist, I have to admit: it’s the artery that calls the shots.”
Stamp Out All Disease?
Dr. Weyand then focused her discussion on management strategies. “We used to look at [large vessel] vasculitis as a transient disease,” she reflected. “After diagnosis, we would treat for two years, and [conclude that] it’s gone. Trust me. It’s not gone.” Although IL-6- and IL-17-dependent inflammation is easily controlled by corticosteroids, the IL-12 interferon-dependent cascade maintains the chronicity of the disease. “We really do not touch the interferon gamma cells with our ‘magic potion,’ the corticosteroids,” she said. As demonstrated by a key 2011 study, by Dr. Weyand and colleagues, there may be potential immunosuppressive strategies through targeting of the NOTCH signaling pathway.1

In the meantime, Dr. Weyand advised clinicians, “Be sure the diagnosis is correct, and be sure [the] patient really needs that treatment and that you can accomplish something.”
During the Q&A that followed, she continued this theme of careful diagnosis and careful consideration regarding treatment: “Do we really need to immunosuppress patients to the point that they have no immune competence left? Intuitively, we may say, of course, we always want to treat inflammation. But we have to be careful, and we have to be aware that we will pay a price for that treatment. If we treat the disease more aggressively, we will have to suppress interferon gamma—but IFN gamma is the major cytokine that protects us from cancer. So we’ll have to be prepared: What’s the price that we are paying, and are we willing to pay that price? And I think that’s the important question. There is no evidence right now that if you were to stop the disease entirely that the prognosis of our patients would improve. For most of our patients, longevity is not reduced by GCA, unless the patient has aggressive aortitis.”
Gretchen Henkel is a medical journalist based in California.
