Prior to the widespread use of bisphosphonates for the management of osteoporosis, multiple myeloma and metastatic cancer to the skeletal system, osteonecrosis of the jawbones was an infrequent condition seen after radiation for oral cancers (osteoradionecrosis) and in chronic odontogenic infections.1
Since the mid-2000s, osteonecrosis of the jawbones has been noted to occur as a result of taking bisphosphonates.2 Bisphosphonates have been shown to not only reduce the incidence of osteoporosis-related fractures, but also skeletal-related events in cancer patients, namely fractures, bone pain, necessity for radiation and
surgery for bone pain and hypercalcemia.3,4 The terms osteonecrosis of the jaw, bisphosphonate osteonecrosis of the jaw, bisphosphonate-related osteonecrosis of the jaws and anti-resorptive osteonecrosis of the jaws (to include denosumab) have now been replaced by the term medication-related osteonecrosis of the jaw (MRONJ), because use of not only anti-resorptive medications, but also anti-angiogenic agents, such as bevacizumab and sunitinib, may also result in this condition.5-7
At this time, anti-resorptive agent-related osteonecrosis by far outnumbers cases caused by other agents.
In addition to their use for osteoporosis and primary and metastatic bone cancer, bisphosphonates are also used for the management of skeletal conditions, such as Paget disease and osteogenesis imperfecta, as well as sporadically for other conditions that cause extensive osteolysis.
Pathogenesis
Bisphosphonates are either non-nitrogen-containing or nitrogen-containing (heterocyclic or alkylamino) agents.9 Alkylamino bisphosphonates (e.g., alendronate and pamidronate) are 100 times more potent that non-nitrogen-containing bisphosphonates (e.g., clodronate and etidronate). Zoledronic acid is the most potent amongst the heterocyclic nitrogen-containing bisphosphonates.
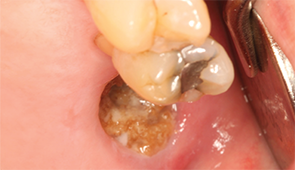
Figure 1: Necrotic
bone in non-healing extraction socket.
Bisphosphonates are potent inhibitors of osteoclast-mediated bone resorption. They bind to hydroxyapatite, and approximately 60% of the absorbed oral dose is deposited into the bone.9 During bone remodeling, bisphosphonates are released from the hydroxyapatite and taken up by osteoclasts. Non-nitrogen-containing bisphosphonates are converted to adenosine triphosphate analogs and accumulate in the osteoclast, leading to markedly reduced bone resorption and bone turnover.10
Nitrogen-containing bisphosphonates inhibit farnesyl pyrophosphate synthase through the mevalonate pathway, also leading to osteoclast apoptosis.11
The theory that emphasizes reduced bone turnover as the primary etiopathogenetic event is also sometimes termed the Inside-Out theory. The theory is that bone necrosis initiates within the bone (whether or not exacerbated by micro-trauma) and spreads outward, to be ultimately exposed. This is well illustrated in an animal study by Allen and Burr.12 In addition, bisphosphonates inhibit the angiogenic response induced by the vascular endothelial growth factor (VEGF).13
Bisphosphonates are also directly toxic to epithelial cells and breakdown of mucosa (as seen in esophagitis).14 In the oral mucosa, this may lead to bacterial ingress (with Fusobacterium nucleatum, a periodontal disease pathogen, playing an important role) and colonization, inflammation and subsequent bone necrosis.15 Animal models have shown that the epithelium is injured and soft tissue healing is poor.14,16,17 Clinical studies have also shown impaired wound healing after dental extractions.18 This theory, based on mucosal breakdown and subsequent damage to, and necrosis of, the underlying bone, is sometimes termed the Outside-In theory.
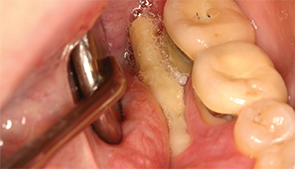
Figure 2: Spontaneous
necrosis of the
mylohyoid ridge.
Other adverse effects associated with the use of bisphosphonates include gastrointestinal upset, acute-phase reactions, renal toxicity (primarily with use of intravenous bisphosphonates), and atypical fractures involving sites not usually seen in osteoporosis and stress fractures.19
Other medications have been implicated in the development of MRONJ, such as denosumab, a fully humanized monoclonal antibody used as an alternative medication to bisphosphonates.20,21 Denosumab binds to the receptor activator of NFkB ligand (RANKL), preventing activation of RANK and inhibition of maturation of pre-osteoclasts to osteoclasts.22 Compared with bisphosphonates, which have a half-life of approximately 10 years, denusamab has a half-life of 25–32 days.
Anti-angiogenic agents, such as anti-VEGF prescribed for the management of metastatic cancers, but also essential for bone remodeling and wound repair, are also associated with an increased risk of MRONJ.6,23 Anti-VEGF agents include small molecule tyrosine kinase inhibitors (TKIs), such as sunitinib, that block the VEGF receptor, and monoclonal antibodies, such as bevacizumab, that bind to VEGF and reduce its biological activity.24 Both agents are used to treat patients with metastatic solid tumors, and both have been shown to cause MRONJ independent of use of anti-resorptive agents.25-27
Although two case reports have suggested that MRONJ developed in patients treated with everolimus (an mTOR [mammalian target of rapamycin] inhibitor), both cases had also been previously treated with zoledronic acid and no cases of MRONJ caused by mTOR inhibitors alone have been reported.28,29 As such, a causal role of mTOR inhibitors and development of MRONJ is not established at this time.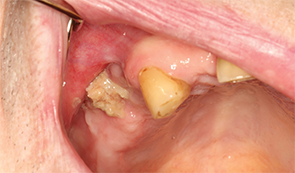
MRONJ is seen in only a small percentage of patients receiving these medications, and this may be a result of genetic differences. In a large, genome-wide association study, four single nucleotide polymorphisms (SNPs) were significantly correlated with the incidence of MRONJ, namely rs1934951, rs1934980, rs1341162 and rs17110453.
In another study among multiple myeloma patients treated with zoledronic acid or pamidronate, the authors found 10 known SNPs involved in bone turnover associated with MRONJ.30 The genes included COL1A1, CYP2C8, MMP2, OPG, OPN, RANK and TNF.31
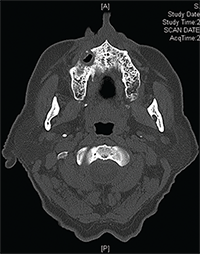
Figures 3A & B: Maxilla involved by osteonecrosis: (top) necrotic bone of right maxilla; and (bottom) bone defect of right maxilla with maxillary sinus involvement.
Another genome-wide association study showed that individuals with an SNP in the RBMS3 gene (associated with collagen formation and bone density) were approximately six times more likely to develop MRONJ.32
Prevalence
Bisphosphonate use is associated with MRONJ in both patients on cancer therapy (frequency of 1–7%) and those with osteoporosis (frequency of 0.02%).33,34 Denosumab use has also been associated with the development of MRONJ in patients with cancer at a frequency of 0.7–1.9%.35 In patients who received denosumab for osteoporosis, the risk for MRONJ is significantly lower (0.04%).36
The risk for MRONJ in patients with cancer on bevacizumab is 0.2%, and increases to 0.9% when used in combination with bisphosphonates.23 There are only a few case reports of MRONJ developing in patients with metastatic renal cell carcinoma treated with sunitinib with no exposure to bisphosphonate therapy.25,26,37
However, the incidence may be as high as 29% in patients with renal cell carcinoma treated with both sunitinib and zoledronic acid.38 Finally, MRONJ has been noted in 1.4% of patients on cabozantinib (a TKI with anti-VEGF receptor) for treatment of metastatic medullary thyroid cancer.39,40
Risk Factors
The single most important risk factor for MRONJ is the cumulative dose of bisphosphonate (because of its long half-life) and, therefore, to some extent, the duration of therapy. In patients with cancer exposed to zoledronic acid or denosumab, the incidence of developing MRONJ increases over time (0.6% and 0.5% at one year, 0.9% and 1.1% at two years, and 1.3% and 1.1% at three years).41 Intravenous zoledronic acid is associated with a higher risk, but this is likely because this is primarily used in cancer therapy and dosed monthly (4 mg) at least for the first two years, and IV bisphosphonate use in osteoporosis is dosed annually, although at a 25% higher dose (5 mg).42,43 As such, duration of use is not as accurate a measure of risk as total cumulative dose.
The other very important risk factor is local trauma, such as from dento-alveolar surgery (e.g., dental extractions), physiologic trauma especially from anatomic factors, such as protuberant tori, and exostoses, although some cases are idiopathic. MRONJ following a tooth extraction in patients with cancer or Paget disease receiving IV bisphosphonates is less than 1%.44 For patients receiving oral bisphosphonates, the risk of ONJ after a dental extraction is 0.09–0.34%.45 In some cases, extractions expose a preexisting site of bone necrosis that caused the tooth to be painful and loose.
Infections, such as periodontal disease and periapical periodontitis, are other known risk factors.46 Periodontal disease-related bacteria have been isolated from necrotic bone in 53% of MRONJ patients; these include Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Streptococcus sp and Eikenella.47 The presence of these bacteria and their relationship with MRONJ remain unclear. Infections are common in the oral cavity, and only a very thin mucosal barrier separates oral bacteria from the underlying bone. This may explain why such osteonecrosis has been reported only rarely at other bony sites, such as the auditory canal and the finger, and even then, always with concomitant MRONJ.48 Denture wearers are at a higher risk for MRONJ when exposed to zoledronic acid, possibly related to trauma of the denture on delicate mucosa (OR = 4.9).49 MRONJ seems to be more prevalent in women, most likely because of the higher prevalence of the underlying condition for which they receive bisphosphonates (e.g., osteoporosis and metastatic breast cancer). Corticosteroid use has also been associated with an increased risk for MRONJ.46,50
Clinical Findings & Diagnosis
The American Association of Oral and Maxillofacial Surgery (AAOMS) defines MRONJ on the basis of three criteria:
- Current or previous treatment with anti-resorptive or anti-angiogenic agents;
- Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than eight weeks; and
- No history of radiation therapy to the jaws or obvious metastatic disease to the jaws.
Patients present with non-healing extraction sockets with exposed bone, or exposed bone on the mylohyoid ridge, tori, exostoses or elsewhere (see Figures 1 & 2). The mandible is involved more often than the maxilla (73% vs. 22.5%), and patients experience jaw pain in 64–82% of cases.50,51 Some patients may also develop traumatic ulcers of the tongue from sharp bone edges.
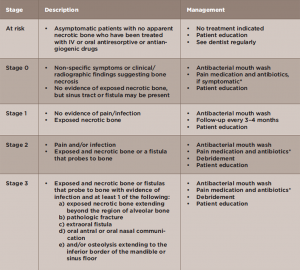
According to the AAOMS, MRONJ is classified into five stages (see Table 1).5 Many maxillary lesions readily become Stage 3, because the posterior teeth lie very close to the sinus, separated only by a thin plate of bone that is easily breached by MRONJ (see Figures 3A–B).
If one presumes that many cases of MRONJ start deep within the bone (osteonecrosis of the jaw, bisphosphonate osteonecrosis of the jaw, bisphosphonate-related osteonecrosis of the jaws and anti-resorptive osteonecrosis of the jaws), exposure of bone is a late stage of this condition. As such, a Stage 0 form of MRONJ (sometimes referred to as non-exposed MRONJ) is recognized where there is no exposed bone, but clinical findings strongly suggest the presence of intra-bony MRONJ, such as extremely loose teeth, sinus tracts, pain disproportionate to the degree of dental disease present, and radiographic findings of moth-eaten bone and sequestra.52 This may involve 14–29% of cases.51,53
Intraoral and panoramic radiographs and cone beam computed tomography (CBCT), a form of focused CT of the jawbones, help delineate the extent of the disease.54,55 Radiographs show poorly demarcated, mottled radiolucencies, with or without sequestra, and often thickening of the lamina dura, the thin shell of bone that surrounds teeth.
Management
Prevention of MRONJ should be the goal and measures should be initiated before or shortly after starting medications that predispose patients to MRONJ.
All patients should be seen by a dentist for a baseline examination and radiographs. This is particularly important for patients with cancer who are higher risk. Because infection is one of the predisposing factors, all dental treatment to reduce and eliminate infection should be completed as quickly as possible, such as having dental restorations placed, teeth cleaned and unrestorable teeth extracted. Even if the incidence of MRONJ is low, such as in the osteoporosis population, removing all sources of dental infection is good practice for the overall health and well-being of the patient.
At this time, anti-resorptive agent-related osteonecrosis by far outnumbers cases caused by other agents.
If the patient requires extraction of a tooth after having been on bisphosphonates for many years, this may be unavoidable because not treating sites of infection also predisposes the patient to MRONJ. There is no absolute contraindication to extraction, and this may be the most appropriate treatment for teeth that are mobile (grade III or higher) and at risk for aspiration, teeth that are in areas of ONJ as determined radiographically and when the patient has continued to have pain and infection despite multiple courses of antibiotic treatment. If the patient has had substantial exposure to bisphosphonate therapy, non-restorable teeth can receive root canal therapy, provided there is no obvious bone disease on the radiographs. Patients should then be followed routinely for regular dental check-ups and dental cleanings.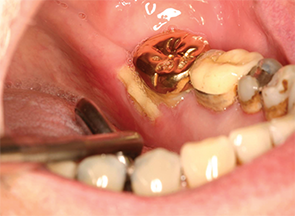
The AAOMS recommends discontinuation of oral bisphosphonates for two months prior to extractions for those patients who have taken an oral bisphosphonate for more than four years or for those patients who have taken an oral bisphosphonate for less than four years with concomitant medical therapy (corticosteroids or anti-angiogenic agents).5 This is controversial because the half-life of bisphosphonates is approximately 10 years, and this would have little effect on bone healing. In contrast, the half-life of denosumab is shorter (32 days), with a terminal half-life of 5–10 days.56
Bisphosphonate use is associated with MRONJ in both patients on cancer therapy (frequency of 1–7%) & those with osteoporosis (frequency of 0.02%).
There are some data that soft-tissue healing may be compromised by bisphosphonates, but this effect is present only for a few weeks after exposure.17 The duration and necessity of a drug holiday after patients have been on anti-resorptive therapies for osteoporosis for years have yet to be clearly elucidated.57
Management of MRONJ depends on disease stage (see Table 1).5 The aims of treatment are to prevent infection and pain in asymptomatic
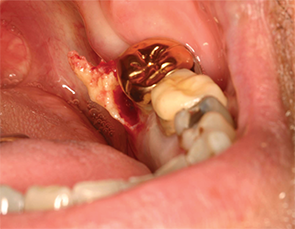
Figures 4A & B: Nonsurgical sequestrectomy: (top) necrotic bone on lingual mandible; and (bottom) removal of fragment of necrotic bone by simple mobilization.
Stage 1 cases, to control pain and infection in Stage 2 cases and to mobilize and remove sequestra in all cases. Stage 3 cases may require surgery and, within the past few years, surgery has been used increasingly and successfully to manage even Stage 2 cases.58
Regardless of the stage, an attempt should be made to gently dislodge the bone fragment (nonsurgical sequestrectomy) at each visit (see Figures 4A–B, left). All patients are placed on an anti-microbial mouth rinse, such as chlorhexidine digluconate. Other treatment modalities include hyperbaric oxygen, laser therapy, ozone therapy, autologous platelet-rich plasma (e.g., VEGF, PDGF, TGF-ß) or teriparatide, although no controlled studies are available for these treatment modalities.59
Adverse effects associated with the use of bisphosphonates include gastrointestinal upset, acute-phase reactions, & renal toxicity.
Studies have investigated bone turnover markers (e.g., C-terminal crosslinked telopeptide [CTx]) both in serum and saliva to assess the risk of developing MRONJ in patients taking bisphosphonates with some promising, but not definitive results.60 Although low serum CTx was considered to be an important marker for bisphosphonate-associated osteonecrosis, this is not specific because after all, low bone turnover markers are an expected and desired outcome in patients given anti-resorptive agents.
Subsequent studies have confirmed the unreliability of this marker for prediction of disease. Although the use of such markers for MRONJ risk is not recommended by the AAOMS, research on this topic merits attention.61,62
Conclusions
There has been much active, ongoing research in the field of MRONJ over the past 12 years since MRONJ was widely recognized, and this has helped us better understand the etiopathogenesis, genetic predisposition, risk assessment, diagnosis of early lesions and management of MRONJ. By working closely together, dentists and physicians can help minimize the occurrence of this condition and treat patients expeditiously if they develop it.
Alessandro Villa, DDS, PhD, MPH, is an associate surgeon in the Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital, and an instructor in the Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston.
Sook Bin Woo, DMD, MMSc, is an associate surgeon in the Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital, and an associate professor in the Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston.
References
- Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: A review. J Am Dent Assoc. 2009;140:864–875.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA. 2006;296:2927–2938.
- Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: A network meta-analysis. Cochrane Database Syst Rev. 2012;5:CD003188.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956.
- Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug related osteonecrosis of the jaw: An uncommon but potentially severe disease. Anticancer Res. 2013;33:1793–1797.
- Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G, 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26:4037–4038.
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045.
- Licata AA. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677.
- Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: Evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44:2201–2210.
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589.
- Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987–994.
- Rabie AB. Vascular endothelial growth pattern during demineralized bone matrix induced osteogenesis. Connect Tissue Res. 1997;36:337–345.
- Wallace JL, Dicay M, McKnight W, Bastaki S, Blank MA. N-bisphosphonates cause gastric epithelial injury independent of effects on the microcirculation. Aliment Pharmacol Ther. 1999;13:1675–1682.
- Mawardi H, Giro G, Kajiya M, et al. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90:1339–1345.
- Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009;45:164–172.
- Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66:839–847.
- Migliorati CA, Saunders D, Conlon MS, et al. Assessing the association between bisphosphonate exposure and delayed mucosal healing after tooth extraction. J Am Dent Assoc. 2013;144:406–414.
- Geissler JR, Bajaj D, Fritton JC. American Society of Biomechanics Journal of Biomechanics Award 2013: Cortical bone tissue mechanical quality and biological mechanisms possibly underlying atypical fractures. J Biomech. 2015;48:883–894.
- Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84:632–637; quiz 638.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone. 2011;48:677–692.
- Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122:181–188.
- Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000–1017.
- Nicolatou-Galitis O, Migkou M, Psyrri A, et al. Gingival bleeding and jaw bone necrosis in patients with metastatic renal cell carcinoma receiving sunitinib: Report of 2 cases with clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:234–238.
- Fleissig Y, Regev E, Lehman H. Sunitinib related osteonecrosis of jaw: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e1–e3.
- Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911–920.
- Giancola F, Campisi G, Lo Russo L, Muzio LL, Di Fede O. Osteonecrosis of the jaw related to everolimus and bisphosphonate: A unique case report? Ann Stomatol (Roma). 2013;4:20–21.
- Kim DW, Jung YS, Park HS, Jung HD. Osteonecrosis of the jaw related to everolimus: A case report. Br J Oral Maxillofac Surg. 2013;51:e302–e304.
- Sarasquete ME, Garcia-Sanz R, Marin L, et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood. 2008;112:2709–2712.
- Katz J, Gong Y, Salmasinia D, et al. Genetic polymorphisms and other risk factors associated with bisphosphonate induced osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2011;40:605–611.
- Nicoletti P, Cartsos VM, Palaska PK, Shen Y, Floratos A, Zavras AI. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: The role of RBMS3. Oncologist. 2012;17:279–287.
- Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: Prior work and current challenges. Osteoporos Int. 2013;24:237–244.
- Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356–5362.
- Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. Int J Clin Oncol. 2014;19:403–410.
- Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: Results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701.
- Koch FP, Walter C, Hansen T, Jager E, Wagner W. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg. 2011;15:63–66.
- Smidt-Hansen T, Folkmar TB, Fode K, et al. Combination of zoledronic acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg. 2013;71:1532–1540.
- Marino R, Orlandi F, Arecco F, Gandolfo S, Pentenero M. Osteonecrosis of the jaw in a patient receiving cabozantinib. Aust Dent J. 2014.
- Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646.
- Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132.
- Grey A, Bolland MJ, Wattie D, Horne A, Gamble G, Reid IR. The antiresorptive effects of a single dose of zoledronate persist for two years: A randomized, placebo-controlled trial in osteopenic postmenopausal women. J Clin Endocrinol Metab. 2009;94:538–544.
- McKeage K, Plosker GL. Zoledronic acid: A pharmacoeconomic review of its use in the management of bone metastases. Pharmacoeconomics. 2008;26:251–268.
- Scoletta M, Arata V, Arduino PG, et al. Tooth extractions in intravenous bisphosphonate-treated patients: A refined protocol. J Oral Maxillofac Surg. 2013;71:994–999.
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65:415–423.
- Tsao C, Darby I, Ebeling PR, et al. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J Oral Maxillofac Surg. 2013;71:1360–1366.
- Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836.
- Salzman R, Hoza J, Perina V, Starek I. Osteonecrosis of the external auditory canal associated with oral bisphosphonate therapy: Case report and literature review. Otol Neurotol. 2013;34:209–213.
- Kyrgidis A, Vahtsevanos K, Koloutsos G, et al. Bisphosphonate-related osteonecrosis of the jaws: A case-control study of risk factors in breast cancer patients. J Clin Oncol. 2008;26:4634–4638.
- Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347.
- Schiodt M, Reibel J, Oturai P, Kofod T. Comparison of nonexposed and exposed bisphosphonate-induced osteonecrosis of the jaws: A retrospective analysis from the Copenhagen cohort and a proposal for an updated classification system. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:204–213.
- Mawardi H, Treister N, Richardson P, et al. Sinus tracts—an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg. 2009;67:593–601.
- Fedele S, Porter SR, D’Aiuto F, et al. Nonexposed variant of bisphosphonate-associated osteonecrosis of the jaw: A case series. Am J Med. 2010;123:1060–1064.
- Treister NS, Friedland B, Woo SB. Use of cone-beam computerized tomography for evaluation of bisphosphonate-associated osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:753–764.
- Treister N, Sheehy N, Bae EH, Friedland B, Lerman M, Woo S. Dental panoramic radiographic evaluation in bisphosphonate-associated osteonecrosis of the jaws. Oral Dis. 2009;15:88–92.
- Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am J Med. 2013;126:13–20.
- Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:85–95.
- Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int J Oral Maxillofac Surg. 2015;44:568–585.
- Kunchur R, Need A, Hughes T, Goss A. Clinical investigation of C-terminal cross-linking telopeptide test in prevention and management of bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:1167–1173.
- Lee CY, Suzuki JB. CTX biochemical marker of bone metabolism. Is it a reliable predictor of bisphosphonate-associated osteonecrosis of the jaws after surgery? Part II: A prospective clinical study. Implant Dent. 2010;19:29–38.
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. Aust Endod J. 2009;35:119–130.