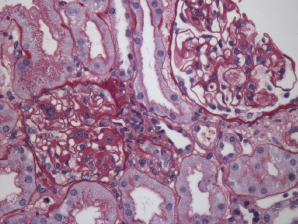
Figure 2. Renal biopsy showing class III lupus nephritis with rare foci of subendothelial deposits (wire loop lesions).
Due to his persistent pancytopenia, the consulting hematologist performed a bone marrow biopsy that did not demonstrate evidence of a malignancy. Within a week of his admission, his creatinine increased to 2.46 (mg/dL) from 0.98 (mg/dL) with a urinalysis that demonstrated mild hemoglobin and albumin. A renal biopsy was performed; it demonstrated evidence of lupus nephritis class III (see Figure 2). Approximately three weeks later, he was also found to have anti-double-stranded DNA antibodies.
The patient was started on prednisone 60 mg daily, hydroxychloroquine 400 mg daily and mycophenolate mofetil 2 g daily.
Two weeks later, the patient developed chest pain and was found to have a small pericardial effusion on echocardiogram, but a normal ejection fraction. That same day, he became confused, and a magnetic resonance imaging (MRI) of the brain was performed; it showed evidence of multiple emboli. Neurology and cardiology were consulted and felt the patient likely had Liebman-Sach’s endocarditis, although there was no lesion found on the valves. The mitral valve was thickened, however.
One day later, the patient developed third-degree atrioventricular block requiring a pacemaker. He was started on anticoagulation given the presence of multiple cerebral emboli. Because of his myocarditis, which was thought to be due to lupus, and a new diagnosis of nephritis, he was switched from mycophenolate mofetil to cyclophosphamide, and all of his symptoms improved. He completed a six-month course of intravenous cyclophosphamide.
He is now taking a remission maintenance regimen of mycophenolate mofetil and hydroxychloroquine, and he remains on anticoagulation, given his history of embolic cerebrovascular events.
SLE is known to cause gastrointestinal symptoms & can occur in the form of … pancreatitis & even malabsorption.
Discussion
SLE is known to cause gastrointestinal symptoms and can occur in the form of serositis, vasculitis, pancreatitis and even malabsorption. Whether or not the malabsorption is from SLE itself or from a concurrent malabsorptive disease can be difficult to discern. Several case reports of concurrent celiac disease and SLE have appeared in the literature over the years. Celiac disease has been reported in other autoimmune diseases as well.
Our patient first presented with symptoms of malabsorption in the presence of an anti-TGA IgA antibody. Anti-TGA IgA antibody is 98–100% sensitive and specific for celiac disease when small bowel atrophy is present, as with our patient.1 However, his atrophy was mild, and the lymphocytes seen could be present in lupus alone. It is possible that the mild atrophy was due to mild malabsorption from SLE itself.