Systemic lupus erythematosus (SLE) can present in many ways and can be difficult to diagnose. Its association with celiac sprue has been only rarely documented, but has appeared in several case reports. When presenting together, it can be difficult to distinguish the underlying disease, because SLE itself has been known to cause malabsorption.
This case report reflects the diagnostic challenges we faced during one patient’s long, complicated hospitalization.
The Case
A 25-year-old, previously healthy, white male presented to our hospital with fever, cough, nausea and vomiting of several weeks duration. He reported a 35 lb. weight loss over the previous year and had recently developed painful oral ulcers. Roughly three weeks prior to admission, he had seen a gastroenterologist for these complaints and was found to have anti-tissue transglutaminase IgA antibodies (anti-TGA IgA) consistent with a diagnosis of celiac disease.
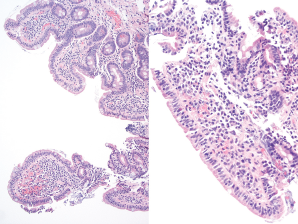
Figure 1. Duodenum biopsy showing partial villous atrophy with borderline increase in intraepithelial lymphoctyes.
An esophagogastroduodenoscopy was performed on an outpatient basis, with findings that could be compatible with malabsorption, possibly due to celiac sprue (see Figure 1). He refrained from eating gluten and his symptoms improved.
He was also undergoing an outpatient evaluation for mild pancytopenia.
At the time of admission, his complete blood count showed the following: white blood cell count 1.9/µL, hemoglobin 12.1 g/DL, and platelets 119 µL. He was found to have an aspartate aminotransferase of 244 (IU)/L and an alanine aminotransferase of 341 (IU)/L. Additionally, he had anti-smooth muscle antibodies, which prompted a liver biopsy that showed only mild steatosis. The consulting hepatologist felt it could be related to his recent diagnosis of celiac disease.
Rheumatology was consulted two days into his hospitalization. He had joint pain in his hands with less than 10 minutes of morning stiffness. On exam, he had dry mucous membranes with three oral ulcers present on the hard palate and a faint red rash on his nose and palms. His breath sounds were minimally decreased without rales or rhonchi. Musculoskeletal exam showed mild synovial thickening of the third proximal interphalangeal joints bilaterally. He was noted to have anti-nuclear antibodies, with a titer of 1:640, in a homogenous pattern. His laboratory tests were also remarkable for a low C3 and C4, and an anti-cardiolipin IgG, which was deemed to be moderately positive. His serum protein electrophoresis was significant for only non-specific hypergammaglobulinemia. His anti-Ro, anti-La, anti-Smith, anti-ribonuclear protein, beta-2 glycoprotein and lupus anticoagulant tests were all negative. During that time, he was mildly hypoxic, and complained of a cough but not dyspnea.
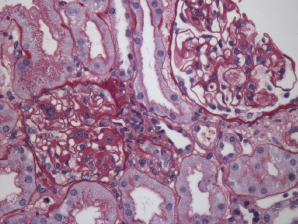
Figure 2. Renal biopsy showing class III lupus nephritis with rare foci of subendothelial deposits (wire loop lesions).
Due to his persistent pancytopenia, the consulting hematologist performed a bone marrow biopsy that did not demonstrate evidence of a malignancy. Within a week of his admission, his creatinine increased to 2.46 (mg/dL) from 0.98 (mg/dL) with a urinalysis that demonstrated mild hemoglobin and albumin. A renal biopsy was performed; it demonstrated evidence of lupus nephritis class III (see Figure 2). Approximately three weeks later, he was also found to have anti-double-stranded DNA antibodies.
The patient was started on prednisone 60 mg daily, hydroxychloroquine 400 mg daily and mycophenolate mofetil 2 g daily.
Two weeks later, the patient developed chest pain and was found to have a small pericardial effusion on echocardiogram, but a normal ejection fraction. That same day, he became confused, and a magnetic resonance imaging (MRI) of the brain was performed; it showed evidence of multiple emboli. Neurology and cardiology were consulted and felt the patient likely had Liebman-Sach’s endocarditis, although there was no lesion found on the valves. The mitral valve was thickened, however.
One day later, the patient developed third-degree atrioventricular block requiring a pacemaker. He was started on anticoagulation given the presence of multiple cerebral emboli. Because of his myocarditis, which was thought to be due to lupus, and a new diagnosis of nephritis, he was switched from mycophenolate mofetil to cyclophosphamide, and all of his symptoms improved. He completed a six-month course of intravenous cyclophosphamide.
He is now taking a remission maintenance regimen of mycophenolate mofetil and hydroxychloroquine, and he remains on anticoagulation, given his history of embolic cerebrovascular events.
SLE is known to cause gastrointestinal symptoms & can occur in the form of … pancreatitis & even malabsorption.
Discussion
SLE is known to cause gastrointestinal symptoms and can occur in the form of serositis, vasculitis, pancreatitis and even malabsorption. Whether or not the malabsorption is from SLE itself or from a concurrent malabsorptive disease can be difficult to discern. Several case reports of concurrent celiac disease and SLE have appeared in the literature over the years. Celiac disease has been reported in other autoimmune diseases as well.
Our patient first presented with symptoms of malabsorption in the presence of an anti-TGA IgA antibody. Anti-TGA IgA antibody is 98–100% sensitive and specific for celiac disease when small bowel atrophy is present, as with our patient.1 However, his atrophy was mild, and the lymphocytes seen could be present in lupus alone. It is possible that the mild atrophy was due to mild malabsorption from SLE itself.
Patients with celiac disease can also have anti-single- and anti-double-stranded DNA antibodies, as well as anti-cardiolipin antibodies.2 In a 2008 study, 2.4% of patients out of 246 biopsy-proven celiac disease patients were found to have SLE, diagnosed anywhere from two to 10 years after the diagnosis of celiac disease had been made.3
Initially, only our patient’s ANA was positive, but three weeks later the double-stranded DNA antibody became positive. His complements were low since the time of our initial encounter. His oral ulcers, transaminitis and subsequent liver biopsy results all could have been due to celiac disease. It was not until his cytopenia worsened, despite an unremarkable bone marrow biopsy, that we started to consider an SLE diagnosis, which was confirmed by renal biopsy.
It was surprising how ill he became over the course of three weeks, even while taking high doses of prednisone. His third-degree atrioventricular block was thought to be due to lupus, although this is very rare. Complete heart block has been reported in only a handful of case reports of adult patients with SLE or Sjögren’s syndrome; interestingly, not all of the patients in these reports had anti-Ro or anti-La antibodies.
This case demonstrates that patients with SLE may present with gastrointestinal complaints or symptoms of malabsorption. Conversely, if a patient with celiac disease presents with signs or symptoms that might be better explained by a diagnosis of SLE, it would be prudent to consider that diagnosis.
 Leslie Pack Ranken, MD, completed her rheumatology training at Wake Forest Baptist Medical Center and serves as an assistant professor in the Department of Internal Medicine at Carolinas Medical Center in Charlotte, N.C.
Leslie Pack Ranken, MD, completed her rheumatology training at Wake Forest Baptist Medical Center and serves as an assistant professor in the Department of Internal Medicine at Carolinas Medical Center in Charlotte, N.C.
References
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992 Jan;102(1):330–354.
- Lerner A, Blank M, Lahat N, Shoenfeld Y. Increased prevalence of autoantibodies in celiac disease. Dig Dis Sci. 1998 Apr;43(4):723–726.
- Freeman HJ. Adult celiac disease followed by onset of systemic lupus erythematosus. J Clin Gastroenterol. 2008 Mar;42(3):252–255.