Febuxostat-induced serum-urate lowering, like that due to allopurinol, was associated with a surge in flares of gouty arthritis when prophylactic colchicine was withdrawn only eight weeks into the therapy after the washout period.5 I advocate starting low-dose prophylactic colchicine two weeks before initiation of urate-lowering therapy. I also advocate maintaining the colchicine regimen for six months in the average patient, and longer in patients with persistent tophi. More intensive serum urate–lowering therapy has the potential to induce more active tophus remodeling, a state that is likely pro-inflammatory at tophaceous deposits.
Uses for Uricase
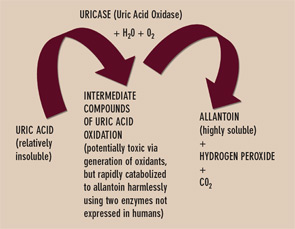
Uricase is one of several enzymes of the final purine degradation pathway in nonprimate mammals and lower primates that catalyzes the conversion of relatively insoluble uric acid to highly soluble allantoin. (See Figure 3) Uricase gene silencing in evolution has rendered the human urate balance delicate. Normal rodents, for example, have a serum urate level of about 1 mg/dL, which is approximately sixfold less than that of normal humans. Humans’ lack of uricase expression may protect them from oxidative stress such as neuronal toxicity of nitric oxide–derived oxidants. Uric acid is the major circulating antioxidant. It was recently demonstrated that the oxidation of uric acid by uricase produces reactive and potentially toxic intermediates in lower mammals that are rapidly degraded by two enzymes that humans also fail to express. Further, the slow, spontaneous conversion of uric acid to allantoin yields the oxidant hydrogen peroxide as a byproduct.
Currently, the recombinant fungal uricase rasburicase is FDA approved for abbreviated, single-course use to prevent tumor lysis syndrome in subjects with hematologic malignancy. This recombinant uricase is neither humanized nor modified by PEGylation. Thereby it is particularly immunogenic and limited by a relatively short half-life. Side effects, including anaphylactic reactions, have been limiting. Robust amounts of neutralizing antibodies to uricase can also develop. PEGylation of uricase promotes less immunogenicity and a longer half-life, and appears better suited for urate-lowering therapy in refractory gout. Indeed, PEG-ylated uricase preparations have induced profound serum urate–lowering in clinical trials. The shift from subcutaneous to intravenous administration of PEGylated porcine recombinant uricase (Puricase) reduced hypersensitivity reactions.6 However, immunogenicity could still constrain safety and longer-term efficacy of current PEGylated uricase preparations in clinical development.


