Innate immunity against invading pathogens involves the distribution of pattern recognition receptors and recognition molecules outside cells, as well as on the cell surface and in the cytoplasm. Innate immune responses to urate crystals that drive gouty inflammation include extracellular crystal-induced complement membrane attack complex assembly, which promotes the CXC chemokine receptor 2 (CXCR2)–binding chemokine expression essential for the neutrophil influx that drives gouty inflammation.9,11,12 (See Figure 4)
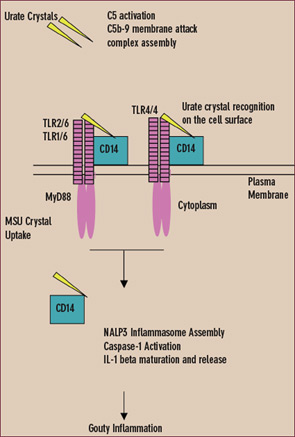
Experimental gouty inflammation is triggered by IL-1 receptor signaling by resident cells at the site of urate crystal injection.13 Macrophage recognition and uptake of urate crystals by the cell surface Toll-like receptors (TLRs) 2 and 4 and by signaling of the shared intracellular TLR adaptor protein MyD88 drives both pro- and anti-inflamamtory cytokine expression.14 Depending on the locus of experimental urate crystal injection, TLR2 and TLR4 expression determines dominant anti-inflammatory effects (such as induction of transforming growth factor b) or pro-inflammatory effects in vivo. However, MyD88, which also is a critical adaptor protein for the IL-1 receptor, is central to both urate crystal uptake by cultured macrophages and urate crystal-induced inflammation in vivo.13,14
In the cytoplasm, urate crystals also employ the cytoplasmic pattern recognition receptor NALP3 (cryopyrin) to drive inflammation.11,12 Upon ligand recognition, NALP3, like other members of the nucleotide-binding oligomerization domain (NOD)–like receptor family, assembles with other proteins in large complexes termed inflammasomes. When NALP3 complexes with caspase-recruiting domain (CARD)–carrying adaptor proteins, activation of the latent inflammatory mediator caspase-1 is stimulated. The activated NALP3 inflammasome complex thereby induces proteolysis of pro–IL-1b and subsequent maturation and release of IL-1b.11
Like TLRs, NALP3 contains leucine-rich repeats.15 Leucine-rich repeat domains of TLRs recognize various pathogen-associated molecular patterns. Therefore, the leucine-rich repeat domain of NALP3 is believed to directly sense components of microbial and other pathogens, stress signals (e.g., high amounts of adenosine triphosphate [ATP] released from dying cells), and urate crystals. Extracellular urate crystal binding of the CD14, an adaptor protein shared by TLR2 and TLR4, does not alter phagocytosis of urate crystals by macrophages, but CD14 switches macrophage encounters with urate crystals that are non-inflammatory into pro-inflammatory events.16 CD14 does so by allowing urate crystals to fully engage the NALP3 inflammasome to activate caspase-1 and induce release of mature IL-1b.16


