Causes
True to autoimmune form, the cause of D2T RA is multifactorial. RA may pose a clinical challenge due to true resistance of disease to DMARDs, or limited drug options due to adverse effects and/or patient comorbidities. Treatment nonadherence is associated with higher disease activity levels, and may lead to inappropriate treatment switches and reduced quality of life.6 And non-inflammatory symptom contributions from fibromyalgia, osteoarthritis, or psychosocial factors associated with poor coping also play a role.7
Management
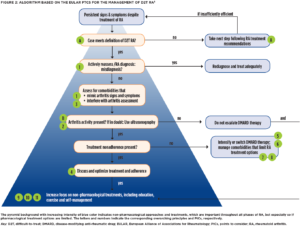
With the problem defined, EULAR developed evidence-based points to consider (PtCs) for the management of D2T RA via systematic literature review and expert consensus (see Figure 2, opposite).8
First, the authors stress two overarching principles: 1) PtCs apply to patients who meet the EULAR definition for D2T RA (see Figure 1, left); and 2) the “presence or absence of inflammation should be established to guide pharmacological and non-pharmacological interventions.” Specifically, “concomitant fibromyalgia, osteoarthritis, and/or psychological conditions, nonadherence, and comorbidities like infections and cancer may contribute to the D2T state” and thus need to be considered when a patient isn’t responding the way we’d like.9
When it comes to differentiating inflammatory from noninflammatory disease activity, ultrasonography can be a useful adjunct to physical exam, but the evidence for biomarkers and other imaging modalities is less convincing.10,11
Next, the authors urge us to consider the possibility of misdiagnosis as a reason for nonresponse to therapy. That is to say, if the patient isn’t getting better despite multiple different therapeutic interventions, take a step back and reconsider the underlying diagnosis. Many conditions, such as crystalline arthritis, vasculitis, cancer and chronic infections, may mimic RA. Professor Nagy shares, “I would say [misdiagnosis] is common in my clinical experience, especially if the patient has seronegative RA or RA that is not clinically typical.”
Treatment adherence should be directly discussed and optimized via shared decision making. “[Assessing nonadherence] is a really essential and complex question. Questionnaires and surveys may help, and serum drug levels might be measured as well—although they generally aren’t part of routine care,” Professor Nagy says.
Another EULAR article provides specific guidance regarding assessment of nonadherence in rheumatic and musculoskeletal disorders.12
As to new bDMARD or tsDMARD selection after failure of two drugs with differing MOAs, the evidence currently doesn’t identify a preferred drug class. The PtCs suggest switching to a drug with a new MOA at the maximum dose deemed effective and safe for the patient. “We need more trials to have evidence-based information regarding the best possible option in different clinical cases,” Professor Nagy says.