Despite an expanding armamentarium of disease-modifying treatments for rheumatoid arthritis (RA), some patients with RA remain symptomatic.1 Current treatment guidelines from both the ACR and the European Alliance of Associations for Rheumatology (EULAR) recommend treat-to-target strategies to achieve remission or low disease activity, and patients want to feel better.2,3 So how can we best help patients with difficult-to-treat (D2T) RA?
In 2020, EULAR took the first steps toward evidence-based guidance for this population, publishing two articles that address the definition and management of difficult-to-treat RA. Here, lead author György Nagy, MD, PhD, DSc, head of the Department of Rheumatology and Clinical Immunology, and professor, Department of Genetics, Cell and Immunobiology, Semmelweis University, Budapest, Hungary, offers insights on what this work means for practicing rheumatology providers and our patients.
Difficult-to-Treat RA Defined
The first step in solving any problem is properly identifying it. By way of results from an international survey of rheumatologists and expert opinion, a multidisciplinary task force created the EULAR definition of D2T RA.4 The task force defined the condition by three mandatory criteria (see Figure 1, below).
Simply stated, D2T RA can be summarized as a patient who has 1) failed two or more biologic or targeted synthetic disease-modifying anti-rheumatic drugs (bDMARDS or tsDMARDs) with different mechanisms of action (MOA) after failing conventional synthetic DMARDs; 2) has active disease; and 3) has poorly controlled disease as perceived by the rheumatologist and/or patient.
If you’re reading these criteria and thinking to yourself, “I could name 20 patients who meet these criteria without even trying,” welcome to the club. An international survey of rheumatologists confirmed the unmet needs of this group.5 These patients are more common than we’d like them to be, especially given the myriad therapeutic options now at our disposal.
Uniform terminology and a clear definition of D2T RA are the first steps toward designing clinical trials to better care for this population. Professor Nagy said, “The definition is necessary to select the appropriate patient population, and our EULAR definition was the first precise characterization of this important patient cohort.”
This definition still needs to be validated, but the Nagy et al. hope the “definition presented here will provide a robust and consistent identification” of these patients, as well as a “platform to define a group of similar patients for research.”
Causes
True to autoimmune form, the cause of D2T RA is multifactorial. RA may pose a clinical challenge due to true resistance of disease to DMARDs, or limited drug options due to adverse effects and/or patient comorbidities. Treatment nonadherence is associated with higher disease activity levels, and may lead to inappropriate treatment switches and reduced quality of life.6 And non-inflammatory symptom contributions from fibromyalgia, osteoarthritis, or psychosocial factors associated with poor coping also play a role.7
Management
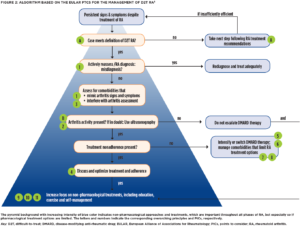
With the problem defined, EULAR developed evidence-based points to consider (PtCs) for the management of D2T RA via systematic literature review and expert consensus (see Figure 2, opposite).8
First, the authors stress two overarching principles: 1) PtCs apply to patients who meet the EULAR definition for D2T RA (see Figure 1, left); and 2) the “presence or absence of inflammation should be established to guide pharmacological and non-pharmacological interventions.” Specifically, “concomitant fibromyalgia, osteoarthritis, and/or psychological conditions, nonadherence, and comorbidities like infections and cancer may contribute to the D2T state” and thus need to be considered when a patient isn’t responding the way we’d like.9
When it comes to differentiating inflammatory from noninflammatory disease activity, ultrasonography can be a useful adjunct to physical exam, but the evidence for biomarkers and other imaging modalities is less convincing.10,11
Next, the authors urge us to consider the possibility of misdiagnosis as a reason for nonresponse to therapy. That is to say, if the patient isn’t getting better despite multiple different therapeutic interventions, take a step back and reconsider the underlying diagnosis. Many conditions, such as crystalline arthritis, vasculitis, cancer and chronic infections, may mimic RA. Professor Nagy shares, “I would say [misdiagnosis] is common in my clinical experience, especially if the patient has seronegative RA or RA that is not clinically typical.”
Treatment adherence should be directly discussed and optimized via shared decision making. “[Assessing nonadherence] is a really essential and complex question. Questionnaires and surveys may help, and serum drug levels might be measured as well—although they generally aren’t part of routine care,” Professor Nagy says.
Another EULAR article provides specific guidance regarding assessment of nonadherence in rheumatic and musculoskeletal disorders.12
As to new bDMARD or tsDMARD selection after failure of two drugs with differing MOAs, the evidence currently doesn’t identify a preferred drug class. The PtCs suggest switching to a drug with a new MOA at the maximum dose deemed effective and safe for the patient. “We need more trials to have evidence-based information regarding the best possible option in different clinical cases,” Professor Nagy says.
Lastly, the PtCs encourage the use of non-pharmacological modalities like exercise, psychological interventions (e.g., cognitive behavioral therapy) and education in all patients with D2T RA.13 Increasing self-efficacy—the ability to control or manage various aspects of their disease—profoundly impacts the well-being of patients.14
Professor Nagy says, “Non-pharmacological therapy, self-management programs and exercise are essential in RA. Clearly, more well-designed trials are needed [in this regard] too.”
High-quality evidence to guide recommendations was scarce, leading to low strength of recommendations. But the Task Force proposed an agenda to help guide further research. Professor Nagy states, “The definition is new. D2T RA is a whole new entity. We hope that there will be significant interest regarding our work, and that our proposal will be used in daily clinical practice and promote further research.”
Conclusion
EULAR has taken an important step forward in improving the care of our patients with D2T RA. A standardized definition may inform future clinical trials, and evidence-based guidance for the care of this population may lead to improved outcomes. Professor Nagy concludes, “This is the major message of our work: avoid overtreatment [and also overdiagnosis])!”

Samantha C. Shapiro, MD, is an academic rheumatologist and an affiliate faculty member of the Dell Medical School at the University of Texas at Austin. She is a member of the ACR Insurance Subcommittee.
References
- de Hair MJH, Jacobs JWG, Schoneveld JLM, et al. Difficult-to-treat rheumatoid arthritis: An area of unmet clinical need. Rheumatology (United Kingdom). 2018 Jul 1;57(7): 1135–1144.
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699.
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021 Jul;73(7):1108–1123.
- Nagy G, Roodenrijs NM, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021 Jan;80(1):31–35.
- Roodenrijs NMT, de Hair MJH, van der Goes MC, et al. Characteristics of difficult-to-treat rheumatoid arthritis: Results of an international survey. Ann Rheum Dis. 2018 Dec;77(12):1705–1709.
- Contreras-Yáñez I, Ponce De León S, Cabiedes J, et al. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci. 2010 Oct;340(4):282–290.
- Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol. 2021 Jan;17(1):17–33.
- Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022 Jan;81(1):20–33.
- Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: Contributing factors and burden of disease. Rheumatology (Oxford). 2021 Aug 2;60(8):3,778–3,788.
- Roodenrijs NMT, Kedves M, Hamar A, et al. Diagnostic issues in difficult-to-treat rheumatoid arthritis: A systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open. 2021 Jan;7(1):e001511.
- Shapiro SC. Biomarkers in rheumatoid arthritis. Cureus. 2021 May;13(5):e15063.
- Ritschl V, Stamm TA, Aletaha D, et al. 2020 EULAR points to consider for the prevention, screening, assessment and management of non-adherence to treatment in people with rheumatic and musculoskeletal diseases for use in clinical practice. Ann Rheum Dis. 2021 Jun;80(6):707–713.
- Roodenrijs NMT, Hamar A, Kedves M, et al. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: A systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open. 2021 Jan;7(1)e001512.
- Taal E, Rasker JJ, Wiegman O. Patient education and self-management in the rheumatic diseases: A self-efficacy approach. Arthritis Care Res. 1996 Jun;9(3):229–238.