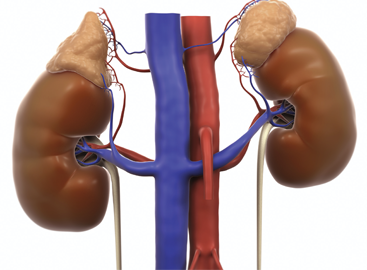
ACTH is a hormone that tricggers the adrenal glands, on top of the kidneys, to produce cortisol, replicating the effects of taking prednisone.
Science Picture Co / Science Source
As rheumatologists, we have a love-hate relationship with the corticosteroid prednisone, a feeling many of our patients share. It’s our most effective medication to quickly shut down an overactive immune system. When we have a patient with life- or organ-threatening autoimmune disease—severe lupus affecting the kidneys or vasculitis causing hemorrhage in the lungs, for example—large doses of prednisone are the most immediate treatment and are considered the standard of care. When a patient has a flare of rheumatoid arthritis, a moderate dose of prednisone can make a remarkable difference within a few days. Among patients with polymyalgia rheumatica, even tiny daily doses of prednisone can make patients with severe muscle pain and stiffness feel normal for years.
But prednisone can also cause significant harm. Taking high doses can raise blood pressure and blood sugar, significantly increasing the risks for cardiovascular disease and diabetes. Long-term use can reduce bone strength, increasing the risk for hip, spine and other fractures due to osteoporosis. In patients with lupus, high doses of prednisone often lead to avascular necrosis (dead bone in the hips and other large joints), a condition that can be remedied only by total joint replacements. And because prednisone suppresses the body’s immune system, taking it can also result in a substantial increase in the risk of mild to serious infections.
A medication that offers the benefits of prednisone without its side effects would be a tremendous breakthrough in rheumatology. This is essentially what every tested immunosuppressant drug is trying to be—thus far unsuccessfully.
Patients, insurers, prescribers & the FDA should mandate large-scale, randomized, prednisone-controlled trials of Acthar prior to allowing further prescriptions for rheumatic indications.
Enter Acthar. Or to be more precise: re-enter adrenocorticotropic hormone (ACTH), albeit in an astronomically expensive form.
ACTH is a hormone released by your brain that triggers your adrenal glands to make cortisol, which is the body’s equivalent of prednisone. The ACTH molecule was discovered in the 1930s by a group of biochemists trying to understand the different chemicals produced by the pituitary gland.1 Interest in using ACTH therapeutically arose after Nobel prize-winning work by Philip Hench, MD, in the 1950s demonstrated the efficacy of cortisol, reconfigured as prednisone, in suppressing immune-mediated inflammation.2 ACTH works by inducing the patient’s adrenal glands to release cortisol, thereby replicating the effect of taking prednisone. Because of this, it very likely has risks and benefits similar to those of prednisone.
The formulation of ACTH now known as Acthar was originally approved by the FDA prior to the 1962 Kefauver-Harris Amendment, which requires that drug developers provide evidence from randomized clinical trials to demonstrate efficacy as a condition of gaining approval for marketing. So Acthar was, as we say, grandfathered in without undergoing a scientifically rigorous evaluation. Because the drug was originally approved for a wide range of autoimmune diseases, the manufacturer does not need to complete trials to demonstrate safety or efficacy.3 In this way, Acthar has indications for a wide range of rheumatic diagnoses: rheumatoid arthritis, lupus, dermatomyositis and polymyositis, sarcoidosis, gout and uveitis.
Acthar is being marketed as the new and improved prednisone, but this characterization is not supported by the facts. Most importantly, it has not been tested head to head with prednisone except for a few small studies on ACTH for infantile spasms. Given this lack of data, we simply have no way to know how the efficacy of Acthar compares with prednisone for the vast majority of indications listed on the drug label. In addition, Acthar’s safety has not been compared to that of prednisone. Patients have told us they are on it because “it isn’t supposed to increase hypertension” like prednisone, but this claim is not justified by the available data or by the expected physiology of the drug.
Now would be a good time to note the tremendous difference in cost between prednisone and Acthar: According to GoodRx.com, a one-month supply of prednisone 60 mg a day (a very high dose) costs less than $25, while a one-month supply of Acthar 80 mg twice a week (an average dose) is around $80,000. Doing the math, that makes Acthar 3,200 times more expensive than the standard therapy, despite a lack of evidence demonstrating any advantage in terms of safety or efficacy.
Jarun Ontakrai / shutterstock.com
Despite this complete lack of evidence and astronomically greater costs, Acthar is being prescribed by a small but dedicated group of clinicians. Recently, a team of researchers and concerned clinicians (three of whom personally experienced high-pressure selling tactics by the drug’s manufacturer) used Medicare and Medicaid data to investigate the frequency of use and overall expense of Acthar. Their study, published in JAMA Network Open, found that in 2015 only 300 providers wrote more than 10 prescriptions for Acthar.4 A total of 235 of these providers were rheumatologists, neurologists, or nephrologists. And out of these 235 specialists, 88% received some sort of compensation from the manufacturer. While many received nominal amounts (less than $200), the top 20% each received over $10,000. There was also an association between receiving higher compensation and writing more Acthar prescriptions—and the Acthar prescriptions written by these frequent prescribers accounted for $200 million in Medicare spending during the period that the study examined. This study also found that more than $1.3 billion had been spent on this drug for several thousand Medicare patients from 2011–2015.
Given the marketing of the drug and the increase in the number of small, open-label studies published since 2015, we suspect these numbers are even higher now. We also know from personal experience that Acthar’s manufacturer is actively looking for clinical researchers open to performing more, small, open-label, nonrandomized trials of their drug.
It’s true that maintaining an active academic research agenda often means that physicians need to solicit and accept grant support and consulting work from pharmaceutical companies. However, it is the responsibility of all clinicians to ensure that they are not overly influenced by this funding, and that they can still objectively weigh the risks, benefits, and financial impact of all treatment decisions. Just because a drug can be prescribed, doesn’t mean it should be prescribed.
Instead of running multiple small unblinded and nonrandomized studies, a much better use of funding would be to conduct large analyses of Medicare and other health datasets to compare the risks and benefits of comparable patients treated with Acthar and prednisone. Patients, insurers, prescribers, and the FDA should mandate large-scale, randomized, prednisone-controlled trials of Acthar prior to allowing further prescriptions for rheumatic indications. These are the studies required for any new immunosuppressant, and the high expense of these trials is used to justify the high costs of the drugs. Drug manufacturers should not be able to aggressively market expensive medications without investing in the kinds of trials that could clarify the drug’s risks and benefits.
So, please, don’t get us started on Acthar. And please don’t start your patients on the drug, either—at least not until a large, randomized, high-quality prednisone-controlled trial has been done to justify the astronomical cost of this more than 80-year-old drug.
Megan Elizabeth Bowles Clowse, MD, MPH, is an associate professor of medicine in the Division of Rheumatology and Immunology at Duke University School of Medicine. She is a clinical associate director of Duke Forge, where health data is put into action. Her clinical research focuses on the management of rheumatologic diseases in pregnancy. She is the director of the Vasculitis-Pregnancy Registry. Additionally, she has collected prospective pregnancies in women with rheumatic disease in the Duke Autoimmunity in Pregnancy Registry, which includes clinical data, pregnancy outcomes and a large sample repository. She received her MD from Vanderbilt University and her MPH at the University of California, Los Angeles.
David Leverenz, MD, is pursuing a fellowship in rheumatology and immunology at Duke University School of Medicine.
Disclosures
Dr. Clowse has received funding from the following sources:
- Consulting: UCB, Regeneron, AstraZeneca, BMS
- Investigator-initiated grants: Janssen, Pfizer
- Independent Medical Education grant: GlaxoSmithKline
Dr. Leverenz has nothing of relevance to disclose.
References
- Collip JB, Anderson EM, Thomson DL. The adrenotropic hormone of the anterior pituitary lobe. Lancet. 1933 Aug 12;222(5737):347–348.
- Glyn JH. The discovery of cortisone: A personal memory. BMJ. 1998 Sep 19;317(7161):822.
- Pollack A. Questcor finds profits, at $28,000 a vial. The New York Times. 2012 Dec 29.
- Hartung DM, Johnston K, Cohen DM, et al. Industry payments to physician specialists who prescribe repository corticotropin. JAMA Network Open. 2018 Jun 29;1(2):e180482.
Editor’s note: Reprinted from Duke Forge by permission.