There are convincing data that endogenous TLR4 ligands may also contribute to the pathogenesis of RA. RA synovial fluids are capable of activating human embryonic kidney cells that are engineered to specifically respond to TLR4 ligands.30 Additionally, the constitutive expression of inflammatory cytokines by RA synovial tissue explant cultures was suppressed by a TLR4 inhibitor. These observations suggest the presence of endogenous TLR4 ligands, capable of driving inflammation in the synovial tissue and fluid of patients with RA. Although studies employing fluids or tissues from patients with RA have not functionally identified a specific pathogenic endogenous TLR4 ligand, tenascin C has emerged as an interesting candidate.
Tenacin C is an extracellular matrix glycoprotein that is not generally expressed in adult tissue but is highly expressed in the rheumatoid joint. Tenacin C, and specifically the region of the molecule called the fibrinogen-like bulb, mediates macrophage activation and induces proinflammatory cytokine production when incubated in RA synovial tissue explant cultures.31 Interestingly, mice deficient in tenascin C, following the induction of immune complex–mediated arthritis, exhibit a more rapid resolution of the arthritis compared with mice that expressed tenascin C, suggesting that the presence of tenascin C promotes ongoing inflammation.
Relevance and Potential Therapeutic Strategy
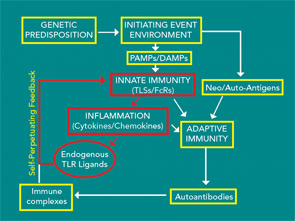
Together, these observations suggest that environmental exposure results in the generation of citrullinated neo-antigens and in the genetically susceptible individual possessing a SE, preclinical disease-associated antibodies are generated. As the repertoire expands, these antibodies appear to result in the activation of innate immunity identified by the production of proinflammatory cytokines and chemokines. By mechanisms yet to be defined, this response becomes apparent in the joints, and clinical disease begins. The induced inflammatory response appears capable of inducing endogenous TLR ligands such as gp96, Snapin, and tenascin C, which may be capable of promoting progressive inflammation, potentially leading to joint destruction. The clinical observation that treating early disease is more effective than treating disease once structural joint damage has occurred may be related to the induction of endogenous TLR ligands, which results in a self-perpetuating process (see Figure 2). At what point in the clinical course endogenous TLR ligands might contribute to the process is not clear. Studies of the synovial tissue of patients with early RA generally cannot be distinguished from those with long standing disease. However, the presence of potential endogenous TLR ligands has not been examined early in the disease.
Recent studies suggest a number of potentially important endogenous TLR ligands. Therefore, neutralization of individual molecules does not appear to be a realistic approach. Strategies that target TLR2 or TLR4, employing neutralizing monoclonal antibodies or even recombinant TLR molecules, fashioned after etanercept, might be possible. In fact, the extracellular domain of TLR2 was effective at suppressing TLR2-mediated inflammation.32 Additional methods to target these pathways include the use of inhibitors that bind the extracellular domain of TLR2 or TLR4, such as has been used in explant cultures.26,30 Additionally, small molecule inhibitors of the Myd88/IRAK pathway might selectively block TLR signaling, leaving other pathways intact. Supporting the value of targeting the TLR signaling pathways, a preliminary study employing a small molecular-weight heat shock protein called chaperonin 10, an inhibitor of TLR signaling, was effective and safe in a small group of patients with RA.33 Together, these observations suggest that there is a Toll that is paid in the persistent and progressive inflammation and joint destruction observed in RA. the rheumatologist

