A Novel Coronavirus Pandemic
The new coronavirus outbreak, COVID-19, reminds us how we have struggled to keep ahead of mutating pathogens through the ages. The mortality rate for COVID-19 infections appears to be 1–3%, somewhere between the 2009 H1N1 influenza outbreak (0.02%) and the 2003 severe acute respiratory syndrome coronavirus (SARS-CoV) epidemic (15%).1
With the highly contagious nature of COVID-19, reported deaths attributable to the virus will likely significantly exceed combined deaths from these preceding epidemics. Much of the effort to halt the spread and harm of COVID-19 is focused on vaccine development and novel antiviral therapies, as was the case with recent Ebola virus outbreaks.2,3
Vaccine development is desirable, but previous efforts to develop a safe vaccine to protect human hosts from the SARS-CoV and MERS-CoV proved challenging. Although vaccines comprising viral peptides were able to induce potentially neutralizing antibodies, in vaccine development studies for both viruses, significant lung immunopathology was observed in test animals following viral challenge.4,5
Found primarily in the lower respiratory tract, angiotensin-converting enzyme 2 (ACE2) is the predominant human receptor for the SARS-CoV S glycoprotein, and sequence homologies of COVID-19 to SARS-CoV suggest COVID-19 may also infect lower airway cells through ACE2.6
New technologies permit the rapid development and production of test vaccines, but these considerations point to safety concerns that may be inherent in vaccines for pathogens that primarily target the lower respiratory tract. Moreover, vaccine development does not address the immediate needs for the significant numbers of patients becoming critically ill in the current pandemic.
Effective antiviral therapy, as well as vigilance and effective supportive care for complications of coronavirus infections, may prove to be of greater immediate importance.
Effective antiviral therapy, as well as vigilance & effective supportive care for complications of coronavirus infections, may prove to be of greater immediate importance [than a vaccine].
A COVID-19 Cytokine Storm Syndrome
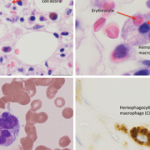
An estimated 20% of individuals infected with COVID-19 are sick enough to necessitate hospitalization, with a subset of patients requiring intensive care.7 Why some individuals become deathly ill and others don’t is unknown, but is likely attributable to host factors. Early reports on the clinical (fever, confusion) and laboratory (hyperferritinemia, lymphopenia, prolonged prothombin time, elevated lactate dehydrogenase, elevated interleukin (IL) 6, elevated C-reactive protein, elevated soluble CD25) features of critically ill patients infected with COVID-19 suggest the presence of a cytokine storm syndrome (CSS) resulting in adult respiratory distress syndrome and multi-organ failure.8-10 Indeed, many of the diagnostic criteria for CSS are reported present in those COVID-19 infected individuals under intensive care (see Table below).11,12
Table: Clinical Features of Hospitalized COVID-19 Infected Individuals & Their Relationship to Cytokine Storm Syndrome Criteria
| HLH-04 Criteria12 | HScore11 | COVID-19 features8-10,24,25 |
| Fever | Fever | Yes |
| Splenomegaly | Splenomegaly | Unknown |
| Hepatomegaly | Unknown | |
| Anemia | Anemia | Yes |
| Thrombocytopenia | Thrombocytopenia | 12% |
| Neutropenia | Neutropenia | Yes |
| Hypertriglyceridemia | Hypertriglyceridemia | Unknown |
| Hypofibrinogenemia | Hypofibrinogenemia | Yes |
| Hemophagocytosis | Hemophagocytosis | Unknown |
| Low NK cell activity | Unknown | |
| Hyperferritinemia | Hyperferritinemia | Yes |
| Elevated soluble CD25 | Elevated soluble CD25 | Yes |
| Elevated serum glutamic oxaloacetic transaminase (GGT) | Unknown, but AST and ALT elevated | |
| Underlying immunosuppression | Some with HIV infection |
Pathophysiology & Genetics of CSS
CSS is believed to occur as a consequence of an accentuated immune response to various triggers, including certain viral infections.13 This was perhaps best modeled in mice with genetic deficiency in perforin, a protein critical to lymphocyte killing of virus-infected cells.
In 2004, Jordan et al. reported that perforin-deficient mice infected with lymphocyte choriomeningitis virus died within two weeks of infection as the result of a CSS.14 Interestingly, mice in which the cytotoxic CD8 T lymphocytes were removed did not completely clear the infection, but survived.14 Similarly, neutralization of the pro-inflammatory cytokine, interferon-g (IFNg), also allowed for survival of these virally infected mice.14 This strongly argues that in this scenario, the death of the mice was dependent on the immune response to the virus rather than the virus itself.
It is noteworthy that mutations in perforin and related genes important for lymphocyte cytolytic capacity were reported in some people who died during the 2009 H1N1 influenza virus pandemic.15 Thus, although we, as a population, are all at risk of infection during viral outbreaks, only a certain percentage of the population may possess genetic risk factors that contribute to frequently fatal CSS.
Treat the Pathogen & the Host
Over the past two decades, substantial progress has been made in understanding the clinical presentation, pathophysiology, risk factors and triggers of CSS, including such disorders as macrophage activation syndrome (MAS) and secondary hemophagocytic lymphohistiocytosis (HLH).16 In addition, a wide array of therapeutic modalities are now available to treat CSS. In the setting of virus-triggered CSS, it is important to treat the infection (if effective antiviral therapy is available), and it may be even more critical to dampen the overly exuberant immune responses responsible for CSS-induced multi-organ dysfunction syndrome (MODS) and death.
Progress in Diagnosis & Treatment
CSS remains a diagnostic challenge, but several criteria have been developed that are not unique to specific inciting triggers or underlying disease.17 Often, a clinical diagnosis of CSS can be reliably and quickly made in presence of fever, hyperferritinemia and multi-organ dysfunction syndrome.18 The earlier therapy for CSS is instituted, the better the outcomes.19
Novel therapies, particularly anti-cytokine approaches targeting IL-1, IL-6, IFNg and IL-18, are now available and have all shown promise in treating various forms of CSS.19-22 IL-1 blockade for CSS has perhaps been the most reported with efficacy for a wide range of triggers, but particularly for rheumatic disease-associated CSS.19
Currently, IL-1 blockade is being studied in a randomized and blinded clinical trial to treat children and adults with CSS [NCT02780583]. IL-6 blockade has been championed for treating CSS as a consequence of chimeric antigen receptor (CAR) T cell therapy for refractory leukemia.22 IL-18 blockade was reported to rescue an infant with a rare genetic autoinflammatory condition complicated by CSS.20 Recently, an anti-IFNg antibody was approved by the U.S. Food & Drug Administration to treat familial hemophagocytic lymphohistiocytosis and is now being tested in secondary forms of CSS as well.21
Lastly, targeting inflammatory cytokine signaling via JAK-STAT inhibition to treat CSS is being reported.23 The relative low risk of co-infections and worsening viral infection with anti-cytokine therapies is mitigated by the benefits afforded by these targeted approaches to treating CSS.
Cytokine-Targeted Therapy
Early reports emanating from China indicate favorable outcomes in a series of severely ill COVID-19 patients treated with a regimen that included therapy with tocilizumab, which leads to blockade of the IL-6 receptor. Moreover, high doses of glucocorticoids (typically available worldwide) are therapeutic options to dampen a detrimental immune response in desperate settings.17
While we are attempting to develop vaccines and trialing novel or repurposed antiviral therapies, let’s also not forget to treat the patient with all we have to offer to help save lives. As rheumatologists, we have much to offer on the front lines to recognize and treat these critically ill individuals with complications of hyper-inflammation.
Randy Q. Cron, MD, PhD, is professor of pediatrics and medicine, and the inaugural holder of the Arthritis Foundation, Alabama chapter-endowed chair in pediatric rheumatology, and the division director and fellowship director of pediatric rheumatology at the University of Alabama, Birmingham. His laboratory studies CD4 T cell transcription as it relates to systemic lupus erythematosus and to HIV-1 infection, as well as genetic mutations associated with macrophage activation syndrome that lead to decreased cytolytic function of lymphocytes.
Winn Chatham, MD, is professor of medicine and the Louis W. Heck Clinical Scholar in Rheumatology in the Division of Clinical Immunology and Rheumatology at the University of Alabama, Birmingham, where he serves as the associate division director for clinical services. In addition to coordinating clinical and translational studies in the UAB Lupus Clinic, Dr. Chatham has clinical interests in disorders of macrophage activation and immunodeficiency-associated autoimmunity.
Acknowledgments
The authors are co-principal investigators of an investigator-initiated clinical trial to study IL-1 blockade for macrophage activation syndrome funded by Swedish Orphan Biovitrum Inc. (SOBI).
References
- Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020 Feb 19 [Online ahead of print].
- Cron RQ, Behrens EM, Shakoory B, et al. Does viral hemorrhagic fever represent reactive hemophagocytic syndrome? J Rheumatol. 2015 Jul;42(7):1078–1080.
- Martinez MJ, Salim AM, Hurtado JC, Kilgore PE. Ebola virus infection: Overview and update on prevention and treatment. Infect Dis Ther. 2015 Dec;4(4):365–390.
- Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016 Sep;12(9):2351–2356.
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012 Sep;12(9): 2351–2356.
- Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020 Jan 23 [Online ahead of print].
- Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 Feb 13 [Online ahead of print].
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020 Jan 30;395(10223):P507–P513.
- Lei C, Huigo L, Wei L, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis. 2020 Feb;43:E005 [Epub ahead of print].
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7.
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014 Sep;66(9):2613–2620.
- Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48(2):124–131.
- Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. 2019 Feb 1;10:119.
- Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004 Aug 1;104(3):735–743.
- Schulert GS, Zhang M, Fall N, et al. Whole-exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J Infect Dis. 2016 Apr 1;213(7):1180–1188.
- Crayne C, Cron RQ. Pediatric macrophage activation syndrome, recognizing the tip of the iceberg. Eur J Rheumatol. 2019 Dec 3;7(Suppl 1):1–8 [Online ahead of print].
- Henderson LA, Cron RQ. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: Diagnosis and management. Paediatr Drugs. 2020 Feb;22(1):29–44.
- Eloseily EMA, Minoia F, Crayne CB, et al. Ferritin to erythrocyte sedimentation rate ratio: Simple measure to identify macrophage activation syndrome in systemic juvenile idiopathic arthritis. ACR Open Rheumatol. 2019 Jul 13;1(6):345–349.
- Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020 Feb;72(2):326–334.
- Canna SW, Girard C, Malle L, de Jesus A, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017 May;139(5):1698–1701.
- Lounder DT, Bin Q, de Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019 Jan 8;3(1):47–50.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014 Oct 16;371(16):1507–1517.
- Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019 Dec;6(12):e630-e637.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):P497–P506.
- Shi H, Han X, Jiang N, Cao Y, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020 Feb 24.
ACR Resource Page: COVID-19
The ACR has created a page with regularly updated resources and brief answers to some frequently asked questions about COVID-19 and SARS-CoV-2, the coronavirus that causes this disease: https://www.rheumatology.org/announcements.