The use of checkpoint inhibitor immunotherapy has changed the treatment for multiple cancers. However, these treatments block the negative regulators of T cell activation responsible for immune homeostasis and, therefore, can result in immune-related adverse events (irAEs) with rheumatic manifestations and musculoskeletal symptoms. Past research has suggested immunotherapy-induced arthritis is an aggressive process requiring high-dose corticosteroids.
To better characterize and understand musculoskeletal irAEs, Melanie H. Smith, MD, PhD, and Anne R. Bass, MD, conducted a retrospective chart review. They identified 10 patients with musculoskeletal irAEs, who received long-term follow up, and were first seen by one of them between 2014 and 2016. All patients had been treated for cancer with immune checkpoint inhibitors at Memorial Sloan Kettering Cancer Center. The research was an attempt to illustrate the required duration of immunosuppressive treatment in these patients. The results were published in the March 2019 issue of Arthritis Care & Research.
The Results
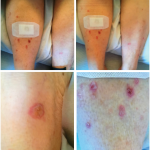
“Patients experiencing musculoskeletal irAEs can have a wide variety of manifestations from oligoarthritis and tenosynovitis, to [polymyalgia rheumatica], to a polyarthritis consistent with [rheumatoid arthritis],” write the authors. “These irAEs had a delayed onset after initiation of immunotherapy, and the majority of patients required prednisone 10–20 mg daily for at least six months to control their symptoms.”
The mean age of the patients was 63.2 years old and 50% were female. For their cancer, seven patients were treated with a combination of checkpoint inhibitors and three with monotherapy. The average time from the first dose of immunotherapy until joint involvement was 6.3 months. Four patients developed inflammatory polyarthritis, four developed oligoarthritis and two developed tenosynovitis. Six patients were anti-nuclear antibody positive, and two had anti-cyclic citrullinated peptide antibodies.
The two patients with the shortest time to the onset of musculoskeletal symptoms either had symptoms prior to initiating immunotherapy or had a strong genetic predisposition. The authors note, “For rheumatologic irAEs, the removal of immune inhibition may unmask native disease, and the delayed onset of symptoms may reflect a preclinical phase similar to that seen in non–immunotherapy-related rheumatic disease.”
Treatment: All 10 patients were treated with systemic corticosteroids, with patients generally advocating for the lowest dose of steroid possible. Six patients achieved good symptom control with 20 mg of prednisone or less daily. Three patients were started on disease-modifying anti-rheumatic drugs, and one patient required a tumor necrosis factor inhibitor (infliximab) to allow steroid tapering.
“In contrast to an earlier case series that suggested that high-dose steroids are required for arthritis control, we found that lower doses of prednisone were usually adequate,” write the authors. Additionally the authors write, “Based on our case series, the duration of musculoskeletal irAEs appears to be much longer [than other irAEs]: The mean symptom duration after stopping immunotherapy was nine months.”
Smith MH, Bass AR. Arthritis after cancer immunotherapy: Symptom duration and treatment response. Arthritis Care Res (Hoboken). 2019 Mar:71(3):362–366.