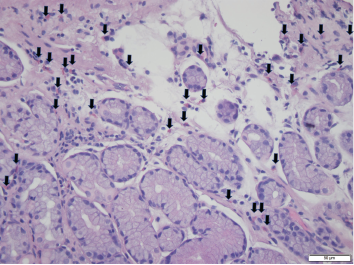
Foci of increased eosinophils (arrows) in superficial layers of the stomach (hematoxylin and eosin stain at 40X magnification).
Eosinophilic gastroenteritis (EGE) is a rare condition caused by eosinophilic infiltration of the gastrointestinal (GI) tract. The condition is subdivided into the GI layers it affects: mucosal, muscular and subserosal.1 EGE usually presents with non-specific GI symptoms, such as impaired motility, intestinal obstruction and, rarely, ascites.2
Below, we report a rare case of EGE leading to eosinophilic ascites associated with rheumatoid arthritis (RA).
The Case
A 57-year-old man with RA presented to an outpatient GI clinic. He complained of abdominal distension and bloating and said he’d been experiencing these symptoms for the past few years. The patient reported passing large amounts of gas, but denied any pain or bleeding with defecation, nausea, vomiting, diarrhea or constipation. He had not experienced any pain or difficulty on swallowing, reflux or burning sensation after meals. He had no fever, chills or weight loss, and was not taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs). His vital signs were stable.
When the patient initially presented with his abdominal complaints, he was taking 20 mg of leflunomide once daily for his long-standing RA. He had previously taken prednisone for his RA flares, but was not on steroids at the time of presentation. He had no surgeries. He did say he was allergic to naproxen, which caused angioedema. He did not smoke, or use alcohol or recreational drugs.
A physical exam revealed a soft, non-tender, distended abdomen with a positive fluid wave. Lab tests revealed normocytic anemia and an elevated erythrocyte sedimentation rate of 76 mm/hr (normal: 0–20 mm/hr), likely secondary to his RA. His white cell count was 5.22 K/uL (normal: 3.50–10.80 K/uL), with a normal eosinophilic count. The patient had previously been worked up extensively at another hospital, where a liver biopsy was performed, but was reportedly unremarkable.
An abdominal ultrasound showed moderate ascites and mild hepatomegaly. A diagnostic paracentesis was performed, which revealed ascites albumin 2.6 g/dL, total protein 4.7 g/dL, glucose 99 and lactate dehydrogenase 178. Serum albumin was 3.66 g/dL (normal: 3.5–5.70 g/dL) . Calculated serum ascites albumin gradient (SAAG) is the current method to determine the etiology of ascites (replaced the outdated method of transudate and exudate fluid classification). We calculated the SAAG to be 1.06 g/dL ([serum albumin 3.66 g/dL] – [ascitic albumin 2.6 g/dL]). This is <1.1, which suggests a peritoneal etiology for this ascitic fluid and rules out portal hypertension (SAAG >1.1). An analysis of the patient’s peritoneal fluid revealed 1,077 white blood cells (0–100/uL), with 28% eosinophils and 29% lymphocytes. There was no evidence of malignant cells, although reactive cellular changes were noted.
A cardiac etiology of the ascites was ruled out with an echocardiogram, which showed an ejection fraction of 65% and no evidence of pericardial effusion. A hepatic etiology to his ascites was thought to be unlikely due to the serum-ascites albumin gradient, normal liver ultrasound, normal liver function tests and prior liver biopsy. Leflunomide was considered a possible cause of the ascites, but upon chart review, the patient had ascites before he started leflunomide therapy. Further, the patient noted that starting leflunomide therapy did not exacerbate his GI symptoms.
Due to the unclear etiology of the ascites, a computed tomography (CT) scan was performed on his abdomen, which revealed dilated small bowel loops, suggestive of small bowel obstruction, mesenteric lymphadenopathy and hepatomegaly. Shortly thereafter, the patient experienced an RA flare and was started on 20 mg of prednisone daily.
A few months later, the patient followed up in our GI clinic and was asymptomatic, with only occasional dyspepsia after eating spicy foods and occasional loose stools. The patient subsequently underwent an esophagogastroduodenoscopy, which revealed gastropathy with atrophic antral mucosa, periampullary diverticulum and patulous pylorus. Random biopsies from the stomach were taken and noted to contain eosinophils throughout, with foci up to 35 eosinophils per high power field (HPF) examination (see Figure 1, left). A colonoscopy proved unremarkable.
At this point, due to the stomach biopsies and ascitic fluid analysis, a diagnosis of eosinophilic gastritis and ascites was reached. Because the patient was asymptomatic and had already been started on 20 mg prednisone daily for his RA, the dosage was not increased. The patient remained asymptomatic at subsequent follow-up.
Discussion
Eosinophilic gastroenteritis is a rare GI disorder characterized by marked infiltration of eosinophils in GI tissue. It can be subdivided into the mucosal, muscular and subserosal types.2 The mucosal type is the most superficial and can present as bleeding or malabsorption. The muscular type can cause wall thickening and motility impairment, leading to intestinal obstruction. The subserosal type is usually the most clinically severe and can be complicated by eosinophilic ascites. The subserosal type usually needs early corticosteroid therapy to manage symptoms.3
No single set of diagnostic criteria exists for EGE. In 1990, Talley et al. suggested three criteria: nonspecific GI symptoms, including peripheral eosinophilia and eosinophilic infiltration of the intestinal wall. In 2018, Yang et al. suggested a histopathological criterion of >30 eosinophils/HPF in >5 HPF or >70/HPF in >3 HPF.4 Another study reviewed the gastric eosinophilic count in normal subjects and suggested the criteria should be >127 eosinophils/mm2 or 30 eosinophils/HPF in at least 5 HPF in absence of known associated causes of eosinophilia.5
In our case, random stomach biopsies showed multiple foci up to 35 eosinophils/HPF in the superficial layers. However, the biopsy was performed after the patient was already taking prednisone for his RA flare. It was thought the prednisone may have partially treated the EGE and possibly blunted the number of eosinophils in the tissue sample.
EGE can rarely cause eosinophilic ascites. Many case reports have shown EGE is associated with peripheral blood eosinophilia, as well as eosinophilic ascites. No diagnostic criteria have been proposed for eosinophilic ascites, and reports range from 23–95% eosinophils in ascites.6-9
The ascites fluid analysis showed 28% eosinophils before the patient started steroid therapy. However, the stomach biopsy that showed 35 eosinophil/HPF was taken after the steroid regimen began for his RA flare. This could have shown a blunted eosinophil count in the tissue because he was already partially treated for his EGE with the prednisone prescribed for his RA. Likewise, although his CT scan showed dilated small bowel loops suggestive of an obstruction, he had no obstructive symptoms or abdominal pain, which may also reflect the fact that he was already being partially treated for his EGE.
The connection between RA and peripheral eosinophilia has been established. However, less is known about RA and eosinophilic infiltration of tissue, particularly in the GI tract. Some case reports have hinted at the connection between EGE and RA.10-14 Most patients showed improvement with steroid treatment (or an increase in steroid dosage if the patient was already on steroids).
Based on our literature search, there is a clear link between EGE and eosinophilic ascites, and a possible association between RA and EGE. To our knowledge, our case report is the first to link RA and EGE leading to eosinophilic ascites.
 Helen Lyo is a fourth-year medical student at SUNY Downstate College of Medicine, Class of 2019, Brooklyn, N.Y.
Helen Lyo is a fourth-year medical student at SUNY Downstate College of Medicine, Class of 2019, Brooklyn, N.Y.
 Eugene Han, MD, is a former gastroenterology fellow at SUNY Downstate Gastroenterology Group of Northern New Jersey, and an attending gastroenterologist.
Eugene Han, MD, is a former gastroenterology fellow at SUNY Downstate Gastroenterology Group of Northern New Jersey, and an attending gastroenterologist.
 Shivakumar Vignesh, MD, is a gastroenterology attending at SUNY Downstate Medical Center, Brooklyn, N.Y.
Shivakumar Vignesh, MD, is a gastroenterology attending at SUNY Downstate Medical Center, Brooklyn, N.Y.
 Nancy Soloman, MD, is a rheumatology attending physician at SUNY Downstate Medical Center, Brooklyn, N.Y.
Nancy Soloman, MD, is a rheumatology attending physician at SUNY Downstate Medical Center, Brooklyn, N.Y.
Acknowledgment
The authors thank Rong Xia, MD, a pathology resident at SUNY Downstate Medical Center for her assistance on this article.
References
- Ingle SB, Hinge Ingle CR. Eosinophilic gastroenteritis: An unusual type of gastroenteritis. World J Gastroenterol. 2013 Aug 21;19(31):5061–5066.
- Klein NC, Hargrove RL, Sleisenger MH, et al. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970 Jul;49(4):299–319.
- Zhou HB, Chen JM, Du Q. Eosinophilic gastroenteritis with ascites and hepatic dysfunction. World J Gastroenterol. 2007 Feb 28;13(8):1303–1305.
- Talley NJ, Shorter RG, Phillips SF, et al. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer and subserosal tissues. Gut. 1990 Jan;31(1):54–58.
- Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: Histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011 Apr;24(4):556–563.
- Bagasarawala I, Maniar JK, Manked A, et al. Eosinophilic ascites—rarest presentation of a rare disease, eosinophilic gastroenteritis. J Assoc Physicians India. 2017 Sep;65(9):93–94.
- Elliott JA, McCormack O, Tchrakian N, et al. Eosinophilic ascites with marked peripheral eosinophilia: A diagnostic challenge. Eur J Gastroenterol Hepatol. 2014 Apr;26(4):478–484.
- Alsulaiman RM. Eosinophilic ascites: A case report and literature review. J Family Community Med. 2015 Sep–Dec;22(3):183–185.
- Aslanidis S, Pyrpasopoulou A, Soufleris K, et al. Eosinophilic enteritis with ascites in a patient with overlap syndrome. Case Rep Med. 2009;2009:734206.
- Al Maksoud AM, Ahmed AS, O’Donnell N. Obstructive eosinophilic gastroenteritis in a patient with rheumatoid arthritis. BMJ Case Rep. 2015 Aug 11;2015:bcr2015210962.
- Paksoy F, Ulaş T, Namal E, et al. Eosinophilic gastroenteritis associated with rheumatoid arthritis. Turk J Rheumatol. 2011 Dec;26(4):321–323.
- Schwake L, Stremmel W, Sergi C. Eosinophilic enterocolitis in a patient with rheumatoid arthritis. J Clin Gastroenterol. 2002;34(4):487–488.
- Medina Perez M, Novales Vasco G, Medina Perez A, et al. Eosinophilic gastroenteritis associated with rheumatoid arthritis: Its presentation as acute abdominal pain. Rev Esp Enferm Dig. 1997 Feb;89(2):143–144.
- Ng WF, Cohen P, Hepburn A, et al. A case of eosinophilic enteritis and rheumatoid arthritis. Rheumatology (Oxford). 2005 Dec;44(12):1585–1586.