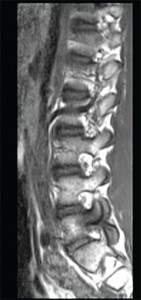
This sagittal T1 weighted MRI image of the lumbar spine shows spondylosis and bone marrowedema within the L5 pedicle extending into the articular facets and lamina.
Imae Credit: Living Art Enterprises/sciencesource.com
ROME, Italy—The explosion of imaging technology has made it more important than ever to establish a standardized way in which imaging can and should be used in clinical practice, an expert said in a session at EULAR 2015, the annual congress of the European League Against Rheumatism (EULAR).
Marie-Antonietta d’Agostino, MD, PhD, professor of rheumatology at the University of Paris, said clinicians now face a staggering array of choices and need guidance. “If you look at the number of imaging techniques that we can use today for our patient management, it’s completely crazy,” she said.
Using an imaging modality in data practice is a balancing act between improvement in outcomes and safety, feasibility and cost. Then there’s the issue of using imaging as an outcome measure, which involves identifying what is to be imaged, the appropriate instrument for assessing that imaging domain and how to use the imaging technique to serve the purpose.
Ideally, the use of imaging in clinical practice should dovetail with its value as an outcomes measure to make it more useful as a research tool, Dr. d’Agostino said.
How to Use Imaging
A EULAR task force on which she sat made recommendations last year describing how imaging should fit into clinical practice—for example, recommending computed radiography, magnetic resonance imaging and ultrasound be used in cases where there is doubt about diagnosis, and indicating that ultrasound and MRI are better than clinical evaluation when it comes to evaluating joint inflammation.1
However, Dr. d’Agostino said there are still some gaping needs in understanding the role of imaging.
More insight is needed, she said, on the importance of subclinical inflammation detectable only by imaging, including synovitis and bone marrow edema, especially when disease activity is low. A greater understanding is needed of the role of imaging in evaluating joint damage. Plus, she said, the feasibility, costs and training needed to use ultrasound and MRI in clinical practice need to be further assessed.
Use It to Guide Care
Paul Emery, MD, Arthritis Research UK Professor of Rheumatology at the University of Leeds, said that with all of the evidence regarding how imaging can help guide care, imaging is practically indispensable to the optimal practice of contemporary rheumatology.
“Imaging can frequently change diagnosis and management,” Dr. Emery said. “And personally, I think management of inflammatory arthritis in the 21st century shouldn’t be without imaging.”
In some cases, a single MRI can leave no doubt that a patient has rheumatoid arthritis, and the image can show a level of disease that makes it clear that methotrexate monotherapy will result in further erosion, Dr. Emery said.
Imaging can determine that what is apparently monoarthritis is actually polyarthritis, or even RA, he said. And in RA patients, when true erosions—erosions along with synovitis—are found on ultrasound, it can mean the difference between someone being diagnosed with undifferentiated inflammatory arthritis and a diagnosis of RA. Imaging can also be predictive: The level of synovitis seen on Power Doppler can predict the likelihood of future erosions.2
Use It to Gauge the Level of Disease
When imaging is used to gauge the level of disease, it can help clinicians tailor their dosing levels, Dr. Emery said.
“What we do with rheumatoid arthritis therapy is incredibly crude,” Dr. Emery said. “We give the same dose of targeted therapy to patients when they have very active disease as we do when they are in remission.” But the dose could be titrated according to disease load if imaging were used for guidance, he said.
Dr. Emery said that if a clinician is trying to predict disease course, imaging is necessary and that it’s helpful in gauging initial response and loss of response to treatment, he said.
Imaging should certainly be done in the 30% to 50% of cases in which there is doubt about diagnosis. But Dr. Emery said he is inclined to go further than that. “I would suggest it’s worth imaging most patients just so you know where you are in terms of baseline disease load,” he said, “certainly if you’re going to give aggressive therapy.”
Georg Schett, MD, professor and chair of rheumatology and immunology at the University of Erlangen-Nuremberg, Erlangen, Germany, discussed how imaging and other techniques might be used to identify patients who might stay in remission when biologic therapy is reduced or discontinued.
An interim analysis of results from an RA study out of his center—called the RETRO study—found that about half of the patients maintained remission after therapy was tapered or stopped. A DAS28 of lower than 2.6 was needed for inclusion.3
“Tapering and discontinuation may be a feasible option in some of our patients in daily clinical practice,” Dr. Schett said.
In the study, researchers found that RA patients with negative ACPA were the subgroup with the highest chance to maintain remission after disease-modifying anti-rheumatic drug tapering.
A study out of Spain found that Power Doppler ultrasound findings can also help shed light on which patients will stay in remission after their drug regimen of biologic therapy is reduced. Synovitis detected by Power Doppler was the strongest predictor of failed biologic therapy tapering in RA patients in sustained clinical remission.4
At Dr. Schett’s center, researchers have looked at the predictive value of multi-biomarker disease activity (MBDA) score and indication of residual disease activity for patients in the RETRO study. They have found that coupling those scores with ACPA positivity strengthens the ability to predict remission after biologic therapy dosing is lowered or stopped.
“The initial evidence suggests,” Dr. Schett said, “that ACPA status, presence of Power Doppler–positive lesions in ultrasound and MBDA scores are potential predictors for relapse of disease after tapering their disease-modifying anti-rheumatic drug treatment.”
Thomas R. Collins is a freelance medical writer based in Florida.
References
- Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013 Jun;72(6):804–814.
- Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004 Apr;63(4):382–385.
- Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: Interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis. 2015 Feb 6. pii: annrheumdis-2014-206439. [Epub ahead of print]
- Foltz V, Gandibakhch F, Ethepare F, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012 Jan;64(1):67–76.

