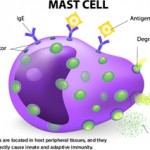
The complement system has long been the scourge of many a medical student (and even a few rheumatologists), although evidence for its importance in normal as well as aberrant immune function is overwhelming. With its multiple pathways and myriad components, regulators, and receptors, this simple little proteolytic cascade (as described by one medical student when compared to immune signaling pathways and all those “kines”) is a major player in innate immunity and instructs the adaptive immune response. Designed to handle bacterial as well as viral infections, especially to block their invasion into the bloodstream, the complement system is also a key participant in the immune and inflammatory response at sites of tissue injury and debris deposition. It is in these arenas that this key participant in innate immunity may turn out to have its greatest impact in clinical medicine.
Thanks to whole genome screens, mutations and polymorphisms have now been identified in protein sequences of complement inhibitory proteins (CIPs), which are key regulators of this system, and shown to predispose to human disease. What is most striking about these genetic studies is the unexpected nature of the disease associations and the role of CIPs in clinically highly disparate diseases that affect both the young and old.
This review will focus on two important examples of complement system dysfunction: one an acute endothelial injury syndrome and the other a chronic inflammatory reaction to biological debris. For both diseases, new and unequivocal evidence demonstrates that the complement system plays a critical role in pathogenesis. These data were initially obtained from whole genome screens and then extended and confirmed by candidate gene mutational screening. We like to call this approach “the genetic hammer” because arguments about the relevance of the genetics and its role in causality are easy to make in the setting of function-altering mutations. Thus, an emerging theme over the past decade is that surprisingly subtle modulation of complement inhibitory function predisposes to microthromboangiopathies, including the antiphospholipid syndrome.1–4
Five years ago, an even greater surprise was the discovery of the association of age-related macular degeneration (AMD) with a polymorphism in a complement regulator.5–8 The seemingly unrelated pathologies of hemolytic uremic syndrome (HUS) and AMD share the common theme of overactivity of the alternative pathway (AP).1–4,9 The breakthroughs in understanding these diseases came from whole genome screens that identified rare variants leading to haploinsufficiency in HUS and a common polymorphism in which the minor allele has reduced function in AMD. These hypomorphs (i.e., genetically based changes resulting in functionally deficient CIPs that control the AP) are present in at least 50% of patients with certain forms of HUS and account for approximately 50% of the attributable genetic risk for AMD. In neither of these diseases was there much preceding evidence for a role for the complement system, although the presence of activation fragments and inhibitors had been well documented in the retina of AMD patients.9

An Ancient System for Host Defense
First identified in human serum in the late nineteenth century as a “complement” to Ab in causing bacterial lysis, the complement system emerged more than a billion years ago as what was probably the first humoral immune system. Coral, sponges, sea urchins, and horseshoe crabs have an AP essentially identical to the one humans possess.10 For example, the addition of endotoxin to hemolymph of the horseshoe crab leads to activation of a protease that then triggers both the clotting and complement cascades.11 Crabs don’t want “bad bugs” in their circulatory system any more than humans do!