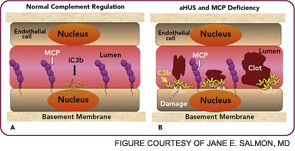
The clinical, genetic, and immunopathologic understandings gained from studying aHUS illustrate how a powerful recognition and effector system, the AP, reacts to altered self. A response to acute injury must be appropriately balanced. Too much activation or too little regulation promotes undesirable tissue damage. More recently, mutations in these same complement regulatory proteins have been identified in preeclampsia, particularly the HELLP syndrome (hemolysis, elevated liver enzymes, low platelets).24,25 Further, patients with SLE and/or anticardiolipin antibody syndrome who developed preeclampsia had a greater frequency of those mutations.26
Chronic Debris Deposition Disease: The Example of AMD
In 2005, four research teams identified a strong genetic link between AMD and a polymorphism in the complement regulator FH.5–8 The lipid/pigment-rich waste known as drusen appears to be a site of an undesirable amount of AP activation (See Figure 5, below).
In 2001, Greg Hageman, PhD, and colleagues demonstrated the presence of C3 fragments, membrane attack complex terminal components, and FH in drusen.9 They proposed a model for AMD pathogenesis based on a prominent role of inflammation. Simply put, according to this model, drusen accumulate, AP is activated, and an excessive inflammatory response damages the retina. Chronic liberation of proinflammatory C3a and C5a anaphylatoxins in the retina are almost certainly deleterious, including their role as a trigger to initiate vascular endothelial growth factor invasion of chorodial vessels (wet AMD) into the macula.27
The polymorphism in FH associated with AMD changes a single amino acid in a region of the protein important for its binding to charged molecules (such as glycosaminoglycans) that are commonly found on cell surfaces and matrices.3 The prevalent hypothesis, for which there is considerable evidence, posits that FH carrying this change (tyrosine in the major allele to a histidine in the minor allele) binds less well to a drusen and therefore results in more AP activation for a given amount of debris. Of great interest, this single amino acid change appears to protect neonates and young children from serious streptococcal infections. Group A beta hemolytic streptococci bind to and coat themselves with FH for protection from complement attack.