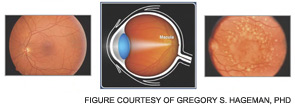
Thus, the interesting conclusion of these studies is that the polymorphic FH variant predisposing to AMD is relatively resistant to binding by this pathogen. This feature protects the neonate and young child against such infections. However, this variant FH doesn’t bind as well to drusen and therefore allows for too much retinal inflammation in AMD in later years. The altered activity of the polymorphic variant may not be the primary cause of AMD, which may be degeneration of retinal elements, but it accelerates the process. Thirty to forty percent of the Caucasian population carries the predisposing polymorphic variant.
As this story dramatically illustrates, evolution has naturally focused on getting humans to a reproductive age, not on vision loss in old age. Polymorphisms of this frequency in the human population usually alter function (selecting for or against) and often relate to enhanced survival in early childhood relative to infectious disease.28
Future Therapeutics
Antibody-based therapeutics are a rapidly growing class of drugs. The U.S. Food and Drug Administration approved the first complement-specific therapeutic, the anti-C5 mAb (eculizumab) in March 2007. This agent has proven to be a highly successful therapy for paroxysmal nocturnal hemoglobinuria (PNH).29,30 In PNH, patient red blood cells are deficient in two membrane complement regulators resulting in a life-threatening hemolytic anemia. This mAb blocks the cleavage of C5 to C5a and C5b and halts complement-mediated lysis of the cells. Several case reports also suggest its efficacy in aHUS and clinical trials are in progress.22,23 Other promising drug candidates and complement-targeted therapeutics are on the horizon.
In summary, the innate immune system reacts to injury and cellular debris. The goal of this response is to facilitate tissue repair (i.e., the removal of debris and the prevention of infection). This interaction must be balanced to avoid undesirable consequences such as a microthrombus in aHUS or choroidal neovascularization (wet AMD) in a retina bearing drusen. Importantly, as these studies of disease show, subtle deficiencies in the function of inflammatory regulators may predispose to untoward consequences. Genetic screens have taught us that, in at least two diseases, dysfunctions of the complement system’s AP contribute significantly to pathologies. It is likely that much variation in disease severity in the rheumatic diseases is explained by rather modest alterations in the control of innate immunity.