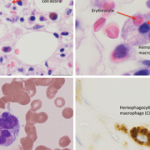
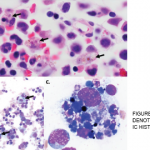
Information about this serious adverse event with lamotrigine was added as a new warning to the prescribing information.
Michele B. Kaufman, PharmD, BCGP, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
ad goes here:advert-1
ADVERTISEMENT
SCROLL TO CONTINUE
References
- Eli Lilly and Company. New release: FDA Advisory Committee recommends the approval of baricitinib 2 mg, but not 4 mg, for the treatment of moderately to severely active rheumatoid arthritis. 2018 Apr 23.
- U.S. Food and Drug Administration. Drug safety announcement: FDA warns of serious immune system reaction with seizure and mental health medicine lamotrigine (Lamictal). 2018 Apr 25.