Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with erosive destruction of diarthrodial joints. Patients who are seropositive are more prone to developing extra-articular manifestations, such as rheumatoid lung, rheumatoid nodules and others. With the development of disease-modifying anti-rheumatic drugs (DMARDs), the incidence and severity of these extra-articular manifestations has declined.
Below, we describe a patient with seropositive RA who developed progressive multifocal leukoencephalopathy after aggressive treatment of rapidly progressive constrictive bronchiectasis with rituximab.
Case
Our patient was a 50-year-old man who was a longstanding patient in our rheumatology practice. Historically, he had been diagnosed with seropositive (+ACPA and +RF) juvenile idiopathic arthritis (JIA) at the age of 12. Treatment early in his disease process included steroids, methotrexate and etanercept. Based on documented disease activity scores, he was in a low disease activity state or remission for the majority of his young life. In fact, he was playing 18 holes of golf and repairing woodwind instruments late into his disease process. Additionally, he had a history of monoclonal gammopathy of unknown significance (MGUS) with IgA lambda light chain seen on serum protein electrophoresis with a normal bone marrow biopsy.
He was well controlled on etanercept until 2012. At that time, he developed a rapidly progressive decompensation in his disease process, complicated by shortness of breath. He had never used tobacco, been exposed to secondhand smoke or been exposed to occupational hazards.
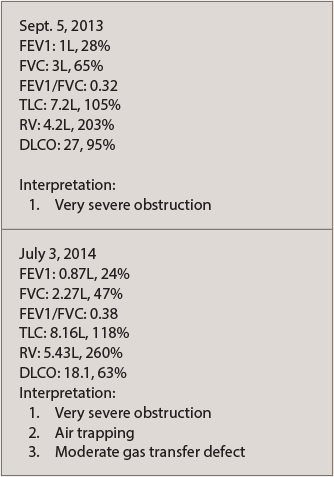
A chest CT scan with contrast noted a left upper lobe subcentimeter nodule that was benign in appearance. A follow-up chest CT in 2015 revealed development of bilateral bronchiectasis, but showed no evidence of interstitial lung disease. He was referred to pulmonology and diagnosed with obstructive lung disease due to rheumatoid arthritis (see PFTs in Table 1).
He was started on conventional medications for chronic obstructive pulmonary disease, which provided only minimal benefit. He was then referred to the Mayo Clinic in Jacksonville, Fla. for a second opinion. He was given the diagnosis of rapidly progressive constrictive bronchiectasis, associated with his RA, in which the smallest airways can become obstructed due to inflammation. This disease is potentially fatal if left untreated. The doctors at the Mayo Clinic recommended against a lung biopsy due to his tenuous pulmonary status.
The etanercept was discontinued, and he was started on an RA-guided regimen of rituximab. He was given a total of two-and-a-half cycles of rituximab before he developed numerous neurological deficits, including left-sided hemiparesis, confusion and ataxia. He was admitted to the hospital for further evaluation.
A brain CT and an MRI (see Figure 1) were not consistent with a stroke. He was diagnosed with possible posterior reversible encephalopathy syndrome (PRES) and was discharged to a rehabilitation facility for physical and occupational therapy.
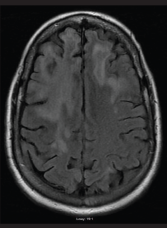
FIGURE 1: An MRI of the brain performed without contrast on June 25, 2016, showed extensive and nonspecific signal abnormalities in the cortical ribbon and subcortical white matter of the right frontal lobe. There is minimal associated parenchymal atrophy in this region without evident edema. No pathologic enhancement, diffusion restriction nor hemosiderin deposition were associated with such signal abnormality.
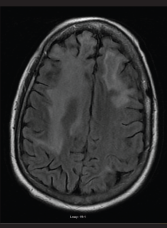
FIGURE 2: An MRI of the brain performed without contrast on July 7, 2016, showed persistent abnormalities in the frontal lobes, which in the right extended into the anterior adjacent parietal lobe. The abnormalities were greater in the right lobe than in the left. Findings in the occipital lobes did not have convincing evidence of restricted diffusion or hemorrhage.
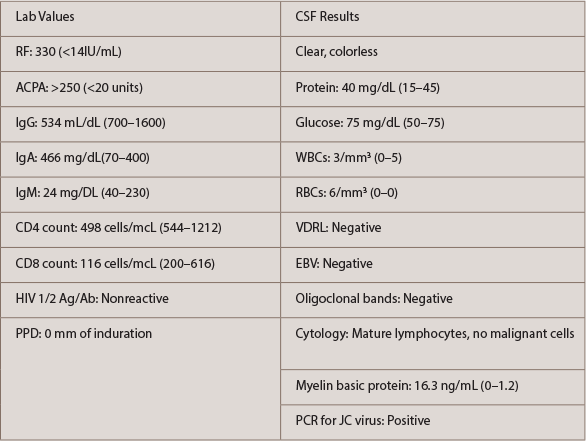
He continued to deteriorate neurologically, which prompted a second neurology consultation. A repeat MRI (see Figure 2) and lumbar puncture were done (see Table 2). Electroencephalogram (EEG) was conducted and was consistent with mild encephalopathy. Laboratory data (see Table 2) supported a hypogammaglobulinemia with low IgG and IgM. IgA was within the normal range. CD4 and CD8 values were low. Cerebral spinal fluid (CSF) results were fairly bland, with the exception of a mild increase in RBCs. Further CSF studies, which were normal, included VDRL, EBV and oligoclonal bands. Cytology of the CSF demonstrated mature lymphocytes without malignant cells present. The CSF did demonstrate a significant elevation in myelin basic protein with a positive PCR for the JC virus.
Our patient was diagnosed with PML based on the above findings. Unfortunately, there are no FDA-approved treatment modalities for this disease process. We discussed potential experimental treatment options with the patient, but we agreed on palliative care alone.
Aggressive physical and occupational therapy were continued, and the patient was discharged home with hospice. He passed away a few months after the initial diagnosis was made.
Discussion
Progressive multifocal leukoencephalopathy (PML) is a rare, fatal, demyelinating disease of the CNS caused by lytic replication of the John Cunningham (JC) virus. The JC virus is ubiquitous in the human body and has an estimated prevalence of 40–70% in the general population.1 The JC virus is usually kept in check by the healthy immune system. However, in the right setting, the virus will invade the CNS and cause an untreatable disease state. The transformation from benign bystander to deadly disease is facilitated by an immunocompromised host, either by infection with the human immunodeficiency virus (HIV) or by immunosuppressive drugs.
Pharmaceuticals that have been associated with the development of PML include natalizumab, infliximab and rituximab. PML is a very rare, but serious, side effect of rituximab and even less frequently seen in those with underlying autoimmune disease.
The risk for developing rituximab-associated PML in those with RA is estimated to be about one in 25,000.2 Most cases of PML associated with rituximab have been reported in those who receive it in combination with other immunosuppressive agents.3 A common example of combination rituximab therapy is when it is used with cyclophosphamide, doxorubicin, vincristine and prednisone. This is known as R-CHOP therapy, a common regimen used to treat non-Hodgkin lymphoma. Specifically, when PML is associated with RA, most patients have received other immunosuppressive agents before receiving the rituximab, such as in this case report.3
Neurologic symptoms of PML are highly variable. These include multifocal deficits that lead to failure to thrive and inevitably to death.
MRI results typical for PML include white matter changes across numerous vascular territories without brain edema. Definitive diagnosis is made by CSF PCR for JC virus or brain biopsy.
As mentioned previously, no FDA-approved therapies for PML exist. Many experimental therapies exist, but none have proved effective. Treatment of an HIV-infected PML patient with antiretroviral therapy was shown to be beneficial but not curative. Since the introduction of newer effective HIV therapies, a decline in the incidence of PML among HIV-infected patients and a decrease in one-year mortality have occurred.4
Conclusion
Biologic DMARDs have forever changed the landscape of RA treatment. With the advent of such therapies comes added responsibility, such as understanding their potential side effects. The cumulative and additive use of immunosuppressive drugs poses a risk for developing opportunistic infections.
In reference to our case, the use of methotrexate, etanercept and rituximab with steroids led to the development of PML. Currently, there are no ACR recommendations for screening RA patients for the JC virus before initiating rituximab. We believe there should be a call to action to develop appropriate prophylaxis and treatment of PML, especially in those who are immunocompromised. We are encouraged by reports of new therapies being developed.5
 Eugene Jalbert, DO, MBA, is a second-year rheumatology fellow at Nova Southeastern University, Largo Medical Center in Largo, Fla. He is from Tampa, Fla.
Eugene Jalbert, DO, MBA, is a second-year rheumatology fellow at Nova Southeastern University, Largo Medical Center in Largo, Fla. He is from Tampa, Fla.
 Priyanka Murali, DO, is a second-year rheumatology fellow at Nova Southeastern University, Largo Medical Center in Largo, Fla. She is from Abu Dhabi, UAE, and grew up in Palm Harbor, Fla.
Priyanka Murali, DO, is a second-year rheumatology fellow at Nova Southeastern University, Largo Medical Center in Largo, Fla. She is from Abu Dhabi, UAE, and grew up in Palm Harbor, Fla.
 Rakhee Shah, DO, completed her rheumatology training at Nova Southeastern University, Largo Medical Center in Largo, Fla. She now serves as a clinical rheumatologist on staff at Suncoast Internal Medicine Consultants in Largo, Fla.
Rakhee Shah, DO, completed her rheumatology training at Nova Southeastern University, Largo Medical Center in Largo, Fla. She now serves as a clinical rheumatologist on staff at Suncoast Internal Medicine Consultants in Largo, Fla.
 Robert DiGiovanni, DO, FACOI, FACR, completed his rheumatology training at the University of Arizona. He now serves as program director for the rheumatology fellowship at Nova Southeastern University, Largo Medical Center in Largo, Fla. He is also president of Suncoast Internal Medicine Consultants.
Robert DiGiovanni, DO, FACOI, FACR, completed his rheumatology training at the University of Arizona. He now serves as program director for the rheumatology fellowship at Nova Southeastern University, Largo Medical Center in Largo, Fla. He is also president of Suncoast Internal Medicine Consultants.
 Rubaiya Mallay, DO, FACOI, FACR, completed her rheumatology training at Nova Southeastern University, Largo Medical Center in Largo, Fla. She now serves as a clinical rheumatologist on staff at Suncoast Internal Medicine Consultants in Largo, Fla.
Rubaiya Mallay, DO, FACOI, FACR, completed her rheumatology training at Nova Southeastern University, Largo Medical Center in Largo, Fla. She now serves as a clinical rheumatologist on staff at Suncoast Internal Medicine Consultants in Largo, Fla.
References
- Lamdhade S, Ashkanani A, Alroughani R. Prevalence of anti-jc virus antibody in multiple sclerosis patients in Kuwait. ISRN Neurol. 2014 Jan 22;2014:861091.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011 Sep;68(9):1156–1164.
- Genentech Rituxan Prescribing Information: Package insert.
- Khanna N, Elzi L, Mueller NJ, et al. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis. 2009 May15;48(10):1459–1466.
- Pavlovic D, Patera AC, Nyberg F, et al. Progressive multifocal leukoencephalopathy: Current treatment options and future perspectives. Ther Adv Neurol Disord. 2015 Nov;8(6):255–273.