A 73-year-old white male presented with a one-day history of a cold, painful, right foot. The foot had a blue discoloration to it, particularly the toes. The emergency physician suspected an atheroembolic cause, given this patient’s age and history of coronary artery disease. However, the patient also reported a one-year history of painful pallor in his digits; therefore, further rheumatologic workup for presumed Raynaud’s phenomenon was requested.
History & Labs
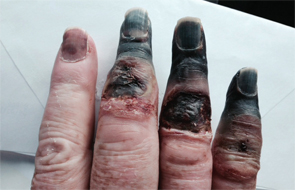
The patient’s necrotic digits on his right hand had to be amputated.
The patient reported that nine months prior to the onset of his current symptoms, he was admitted to this same hospital with similar complaints, namely a cold and painful right foot. In fact, he had been admitted multiple times over the preceding two years for painful, blue fingers and toes, thought to be secondary to microembolic disease with an unknown source.
Each time he presented with these symptoms, he was placed on a heparin drip, and his symptoms spontaneously resolved. Nine months prior, he had been placed on a heparin drip when he presented with a cold and painful right foot. Despite this treatment, on hospital day 3, he suddenly developed cyanosis with necrosis of the third to fifth digits of the right hand, distal to the PIP joints. Thromboembolic disease was suspected, and the patient was found to have a patent foramen ovale that was subsequently closed. A thrombus or vegetation was found on an atrial pacer lead as well.
He was started on warfarin. The necrotic digits of his right hand required surgical amputation, but the right foot symptoms spontaneously improved, and he was discharged home without further sequela.
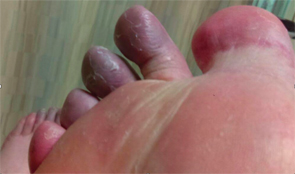
The patient’s first to fourth digits on his right foot had a bluish discoloration, as did his forefoot.
The patient has a remote history of tobacco use (quit 25 years prior to presentation). He notes that during the past one year, several of his fingers would turn white and were painful, especially when exposed to the cold. He was exposed to extremes of hot and cold weather frequently during his 32 years of service in the army and never noticed his hands becoming painful nor turning white, blue or red. He reports that he has had an unintentional 40 lb. weight loss in the past year. He bruises easily, but attributes this to the warfarin prescribed since his admission nine months earlier for presumed microembolic disease.
In addition to coronary artery disease, his past medical history includes coronary artery bypass grafting, ischemic cardiomyopathy with placement of a pacemaker and ICD, resection of a bladder cancer and a small right common iliac artery aneurysm.
The patient had normal pulses bilaterally in all extremities. His first to fourth digits on his right foot had a bluish discoloration, as did his forefoot. His midfoot and fifth digit were red. The rest of his foot appeared normal.
Secondary Raynaud’s was unlikely due to a lack of any additional signs or symptoms suggestive of a particular connective tissue disease. The patient shared photos from his cell phone of his necrotic fingers prior to amputation. An atheroembolic phenomenon causing simultaneous ischemia of three digits all at the same arterial level seemed unlikely, and there was no history of trauma to the arm or hand that would lead one to suspect ulnar artery aneurysm and thrombosis. Cryoglobulinemia was suspected.
Further chart review revealed an abnormal serum protein electrophoresis in 2011 (revealing an IgM monoclonal gammopathy) with no additional follow-up on this test. Cryoglobulins were ordered and were positive. Unfortunately, the patient had been discharged home by the time this test result was known, because he had again experienced spontaneous improvement of his symptoms.
He did return to the emergency department a few weeks later, again complaining of discoloration and pain in the right foot. A serum protein electrophoresis was obtained at this time and again revealed a monoclonal IgM lambda protein. The patient was referred to hematology for a bone marrow biopsy, which revealed 20–30% involvement by a CD20+ B cell lymphoma (negative CD5 and CD10) with a smaller population of CD138 plasma cells.
The patient was diagnosed with lymphoplasmacytic lymphoma (Waldenström macroglobulinemia) complicated by type I cryoglobulinemia. He is being treated with plasmapheresis, rituximab and cyclophosphamide.
Discussion
Waldenström macroglobulinemia is a hematologic malignancy characterized by a lymphoplasmacytic bone marrow infiltration and the presence of immunoglobulin M (IgM) monoclonal protein.1 Usually, patients are elderly and asymptomatic at diagnosis. Constitutional and neuropathy-related symptoms may be present, as well as IgM-induced hyperviscosity-associated features. It is incurable.
Treatment is reserved for patients with severe constitutional symptoms, severe cytopenias, hyperviscosity-related symptoms, symptomatic lymphadenopathy, concomitant AL amyloidosis, IgM-related neuropathy and symptomatic cryoglobulinemia.
 Caitlin Kesari, MD, is a rheumatology fellow at the University of Cincinnati Medical Center. She is a graduate of Northeast Ohio Medical University (Rootstown, Ohio) and Mount Carmel West Internal Medicine Residency (Columbus, Ohio).
Caitlin Kesari, MD, is a rheumatology fellow at the University of Cincinnati Medical Center. She is a graduate of Northeast Ohio Medical University (Rootstown, Ohio) and Mount Carmel West Internal Medicine Residency (Columbus, Ohio).
 Avis E. Ware, MD, is a professor of medicine and the program director for the Rheumatology Fellowship Training Program at the University of Cincinnati Medical Center.
Avis E. Ware, MD, is a professor of medicine and the program director for the Rheumatology Fellowship Training Program at the University of Cincinnati Medical Center.
Reference
- Kapoor P, Paludo, J, Vallumsetla N, et al. Waldenström macroglobulinemia: What a hematologist needs to know. Blood Rev. 2015 Sep;29(5):301–319.