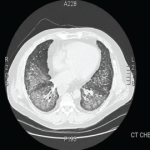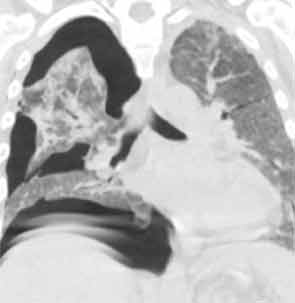
His tracheostomy was decannulated three weeks later. He required pleurodesis for recurrent pneumothorax, but by mid February, the last chest tube was removed. At the time of his discharge to a rehabilitation facility, his oxygen saturation was 95–97% on room air.
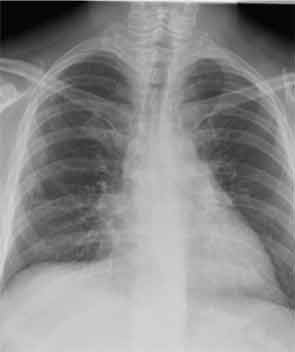
He has since been slowly recovering from critical-illness myopathy. In early March, he was discharged home. Prednisone has been tapered in 10-mg decrements monthly. At the end of March, we saw him back in our outpatient clinic for the first time. He had regained about 30 pounds since hospital discharge. He had nearly full strength in all extremities. He remained B-cell depleted and continued to have a remarkable sustained response to steroids and rituximab in terms of his lung disease. Figure 5 (below) shows his most recent chest X-ray.
Discussion
The antisynthetase syndrome comprises a distinct clinical subset in the polymyositis/ dermatomyositis spectrum in which patients present with the combination of mechanic’s hands, Raynaud’s phenomenon, fever, arthritis, myositis, and interstitial lung disease (ILD).1 Not all of these clinical features have to be present to consider the syndrome.
In our patient, several clinical and laboratory findings provided clues towards a diagnosis of the antisynthetase syndrome. The most intriguing finding on his physical exam was the presence of mechanic’s hands. In retrospect, our patient also had most of the other features of the syndrome, including Raynaud’s phenomenon, fever, arthritis or arthralgias, and ILD. He did not have clinical or laboratory evidence of myositis, although we might have missed subtle muscle weakness given his intubated and sedated status. Another diagnostic clue early on in his evaluation was the presence of positive cytoplasmic antibodies with a negative ANA on immunofluorescence testing. Antisynthetase antibodies localize to the cytoplasm and produce a cytoplasmic staining pattern on immunofluorescence staining for ANAs.2 While a Jo-1 antibody was negative, this prompted us to send a myositis specific antibody panel. The patient, furthermore, had a positive Ro antibody on solid phase assay testing (ELISA).
Ro antibodies comprise antibodies to Ro-52 and Ro-60.3,4 Ro-60 resides in the nucleus but loses its immunoreactivity during preparation of the human epithelial cell line–2 (HEp-2) cell substrate and therefore does not produce a staining pattern on ANA testing using the traditional HEp-2 cell substrate. The result then is a falsely negative ANA. Ro-52, on the other hand, resides in the cytoplasm and may produce a cytoplasmic staining pattern on ANA immunofluorescence testing. Whether the Ro antibody in our patient represents antibody to cytoplasmic Ro-52 or nuclear Ro-60, both of which do not produce nuclear staining on ANA testing, is unclear. We suspect that he has the Ro-52 antibody because anti–Ro-52 antibodies but not anti–Ro-60 antibodies have been detected in a subset of patients with idiopathic inflammatory myositis. His cytoplasmic staining pattern could be the result of the PL-7 antibody alone or the combination of PL-7 and Ro-52.